Playlist
Show Playlist
Hide Playlist
Polypeptides – Peptides
-
03 Basic Peptides.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
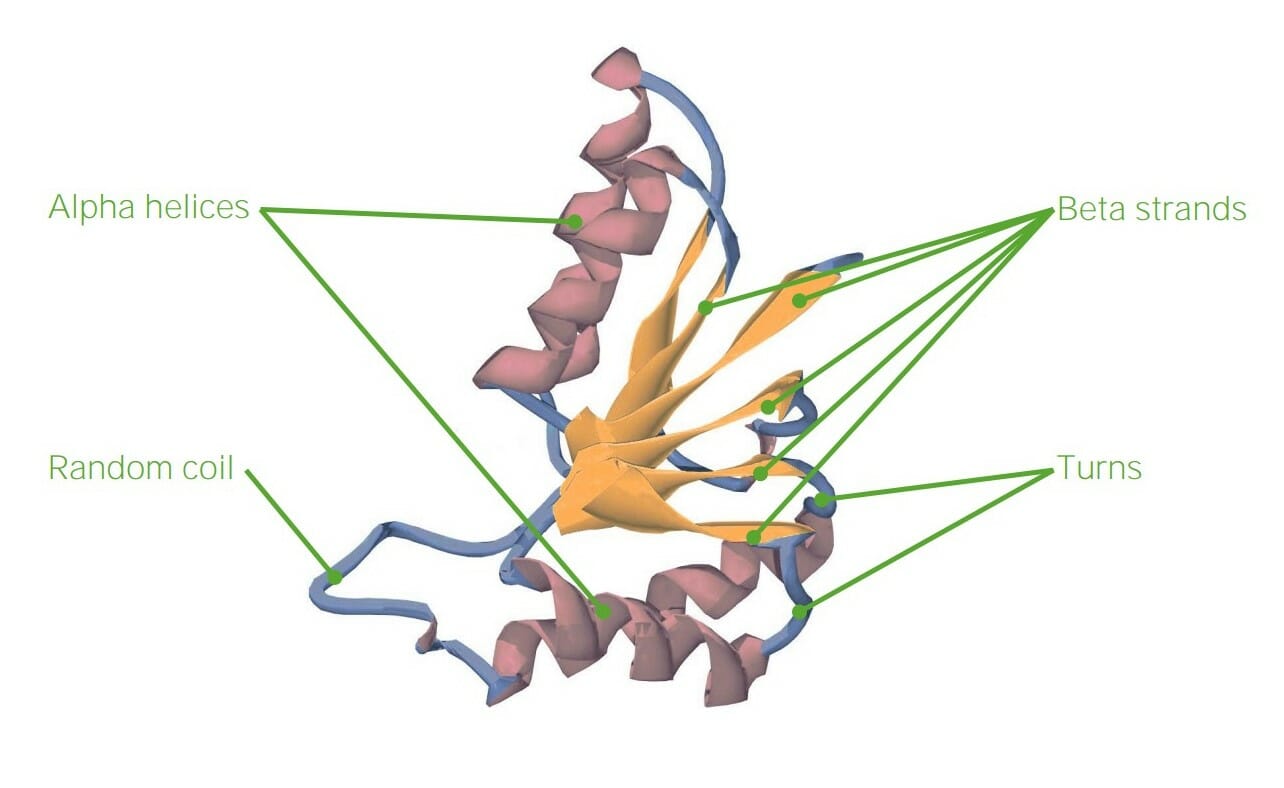
00:00 another way of analyzing or looking at peptide bonds showing in a two-dimensional projection. 00:06 The two-dimensional projection is of a peptide that contains six amino acids. Starting from the left, which is the amino terminus of a polypeptide chain, and moving to the right which is the carboxyl terminus of the peptide chain. We can see that we have the amino acids glycine, phenylalanine, aspartic acid, threonine, alanine and asparagine linked together by peptide bonds. Now we can see that the amino terminus has its name because it's the only portion of the molecule that has a free alpha amine. We can also see that the carboxyl terminus is called that because it's the only part of the molecule that has a free alpha carboxyl group. All of the individual alpha amines and alpha carboxyls are joined together in making the peptide bonds internal to the molecule. Another thing to note on this feature is the position of the R groups relative to each other. Because the alpha carbons are arranged in a trans-configuration, the individual R groups on those alpha amino acids appear to flip, down, up, down, up, down, up moving across the molecule. So starting for example with glycine at the left, we can see that the alpha R group, the alpha carbons R group, which is a hydrogen, is in the up position. In the case of phenylalanine, which is the second amino acid, the R group, which is an aromatic ring, is in the down position. And then going through the rest of the molecule, we see up, down, up, down, up, and again this corresponds to the orientation of the individual alpha carbons. 01:38 Another way of viewing this same thing, is to look at the peptide as a whole now including the single bonds between the alpha carbon and the peptides as shown here. Now the peptide bond as I noted, is a double bond that cannot rotate. But the double bond of the peptide bond is each joined to an alpha carbon. And the alpha carbon is joined to two peptides as seen here. As you look in the slide, you'll notice that each alpha carbon has a little circle around its single bond, indicating that its single bond can rotate. The rotation of the single bond gives some degrees of freedom for the polypeptide chain at that point. 02:19 Now because the peptide bond itself can't rotate, but the individual single bonds around the alpha carbon can rotate, it means that it's important to understand how much freedom there is to rotate those single bonds across the alpha carbon. Now the two bonds, the two rotational bonds across the alpha carbon are measured by angles called phi and psi. The phi rotational angle associated with the bond of the alpha carbon is the one between the alpha amine and the alpha carbon, whereas the psi rotational bond is the one associated between the alpha carbon and the alpha carboxyl group on the other side. 02:57 The Indian scientist named Ramachandran realized that there would be limitations on the rotational angles of phi and psi that would arise as a result of steric hindrance, steric interactions that would happen with the individual atoms that are projecting out from the polypeptide chain. He wrote a computer program to analyze what rotational angles would be predicted to give the most stability, according to positioning, and which ones would give the least stability according to positioning. The plot that he created was given his name, it's called a Ramachandran plot. And it shows the plots of stability superimposed on a graph of 360° of rotation of angle for the psi rotational angles on the y-axis and for phi on the x-axis. 03:47 When the results of Ramachandran's analysis were realized, it was apparent that there were only two major regions of stability that existed within the rotational angles of phi and psi. Now today we know that those angles correspond to common structures we find in proteins known as beta strands, that correspond to the region on top, in dark blue, and the alpha helix which correspond to the dark blue region on the bottom.
About the Lecture
The lecture Polypeptides – Peptides by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following is NOT a component of amino acids that contributes to protein structure?
- Alpha nitrate
- Alpha amine
- Alpha carboxyl
- R-group
What is the name of the rotational angle between the alpha carbon and the alpha carboxyl?
- Psi
- Phi
- Gamma
- Delta
- Epsilon
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Very well explained and detailed lecture, but it would be nice for better understanding to have some names and terms (e.g. Ramachandran plot) displayed in the video!