Playlist
Show Playlist
Hide Playlist
Other Approaches to Beta Lactamase Issue – Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
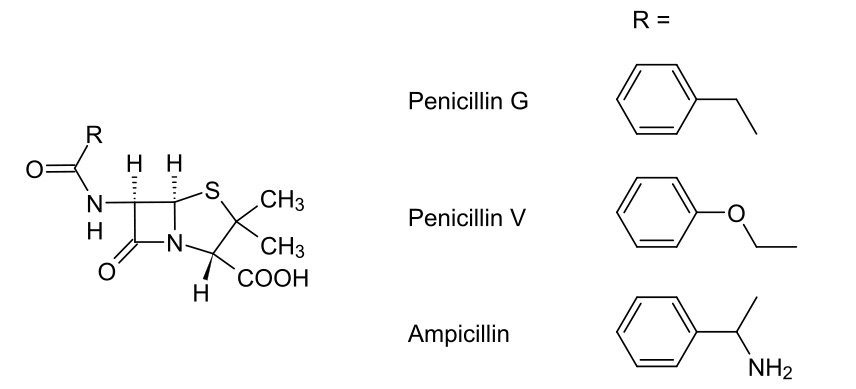
00:00 Other approaches to the problems of bacteria producing these beta-lactamases, which go onto inhibit antibiotics, involves the use of a sacrificial compound which can be given or co-administered with penicillins to treat penicillin-resistant bacteria. An example of this is clavulanic acid. 00:22 Clavulanic acid is a structure shown here has remarkable similarity to penicillin as you can appreciate: has the beta-lactam ring, okay? It doesn’t have the thiazolidine ring, but what it does have is the ability to interact directly with beta-lactamases. So, if you have a beta-lactamase-producing bacteria, which under normal circumstances would mean that it would be resistant to the penicillin that you would administer, you can co-administer this clavulanic acid and use it as a sacrificial compound so that it occupies and irreversibly binds to the beta-lactamase produced. This means that there is insufficient beta-lactamase around to then react with the penicillin that you’ve just administered which you want to work. 01:13 So, the reason that this is used is because, in of itself, it is not an antibiotic, but it irreversibly binds to the beta-lactamase and prevents it from breaking down, therefore, penicillins that you’ve administered. Standard names for these co-administered antibiotics would be things like amoxiclav, a mixture of amoxicillin and clavulanic acid. 01:36 Now, I want to just briefly talk to you about another class of beta-lactam antibiotics which relates to the penicillins we’ve just discussed and they are the cephalosporin class. 01:48 They have been found to be generally more resistant to acid hydrolysis and also generally, more resistant to beta-lactamase produced by bacteria which have this grown resistance. 02:04 As you can see, there’s a slightly different way in which they work, but they both or they all have this characteristic beta-lactam four-membered ring system. 02:14 In this scenario, the serine represented here of the active site of the penicillin-binding protein is shown as a line with OH coming out of it. The lone pair of electrons on the oxygen attacks the carbonyl-carbon, opens it up and cleaves the beta-lactam ring forming, as you can see, an ester bond. There is an entropically driven part for this mechanism which is the loss of an acetate group, as you can clearly see at the other end of this six-membered ring. 02:46 Cephalosporin C, as you can see here, which is one of the first-generation of cephalosporin-based antibiotics, has evenly-distributed Gram-negative and Gram-positive activity. So, generally speaking, this was a subsequent development improvement on the penicillin class using this six-membered ring cephalosporin class.
About the Lecture
The lecture Other Approaches to Beta Lactamase Issue – Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
What is the role of a sacrificial compound in antibiotic therapy?
- These compounds help to combat antibiotic-degrading enzymes produced by the antibiotic-resistant bacteria.
- These compounds form a protective shell around the antibiotic molecule and hence protect it from enzymatic attack.
- These compounds prevent the acidic hydrolysis of the antibiotic molecule by proton quenching.
- These compounds protect the antibiotic-resistant bacteria from antibiotic attack by blocking the antibiotic receptors on the cell surface.
- These compounds inhibit the synthesis of antibiotic-degrading enzymes by bacterial cells.
Which of the following is NOT true about clavulanic acid?
- It contains a thiazolidine ring in its structure that hinders the binding of beta-lactamase to penicillin.
- It acts as a sacrificial compound in the treatment of penicillin-resistant bacterial infections.
- It acts as a mechanism-based beta-lactamase inhibitor by binding irreversibly with the lactamase molecules.
- It is structurally similar to the penicillin due to the presence of beta-lactam ring.
- It does not contain any antibiotic activity by itself.
Amoxiclav is which of the following?
- A mixture of amoxicillin and clavulanic acid.
- A mixture of azithromycin and clavulanic acid.
- A mixture of erythromycin and clavulanic acid.
- A mixture of levofloxacin and clavulanic acid.
- A mixture of norfloxacin and clavulanic acid.
What are cephalosporins?
- Bactericidal antibiotics which exhibit more resistance towards acid hydrolysis and beta-lactamases.
- Fungicidal antibiotics which are susceptible to acid hydrolysis.
- Fungicidal antibiotics which are sensitive to beta-lactamase attacks.
- Virucidal agents which inhibit the beta-lactamase enzymes.
- Algicidal agents which destroy the beta-lactamase enzymes.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |