Playlist
Show Playlist
Hide Playlist
Enzyme Inhibitors
-
Slides 11 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
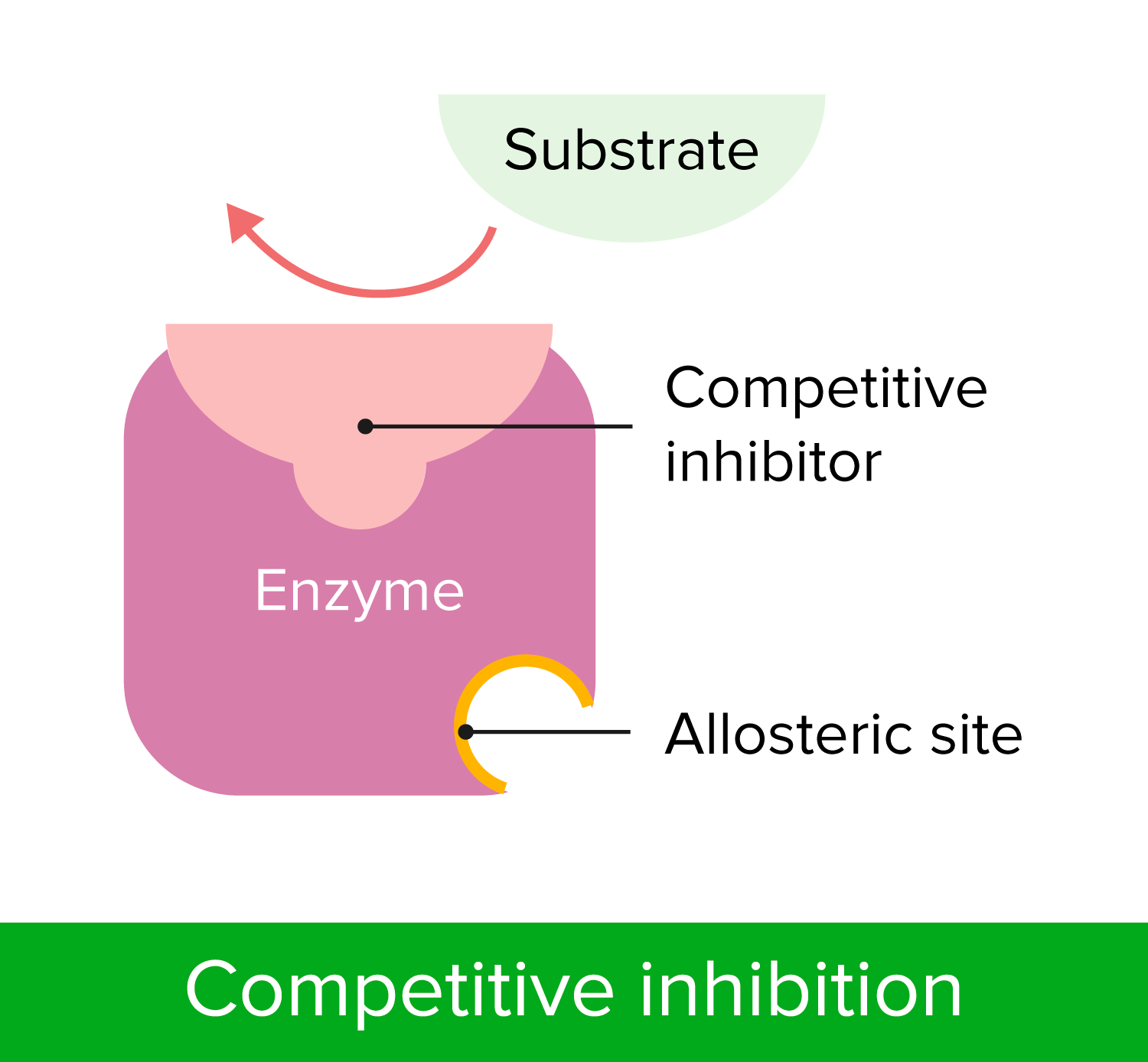
00:01 Enzymes are generally targeted by inhibitors which prevent them from working. These are molecules which combine in the enzyme and prevent substrates or cofactors from binding, as we will see in the final slide for this particular final lecture, for this particular module, where we will be looking at penicillin-binding protein, which is an example of an enzyme found on the surface of bacteria which we can target with antibiotics. 00:29 The reaction which the enzyme would normally catalyse can then not take place. So, how would you go about designing an enzyme inhibitor? Give it some thought based on your understanding of functional group and also shape and size. 00:44 Let’s have a look at how we can inhibit an enzyme, stopping it from working. And I’d like to draw your attention to the concept of reversible inhibition. It’s been found that some enzyme inhibitors block the active site by binding in a similar way to the substrate, that is to say, at the active site. 01:02 So, let’s have a look at two scenarios, where we have our normal substrate, shown as the blue ellipse binding to the enzyme, which will then function. As you can appreciate, if we have an inhibitor, which has a similar affinity for the enzyme, there will be an equilibrium between bound and unbound substrate and bound and unbound inhibitor. 01:26 What this effectively means is if we increase the concentration of the inhibitor, we stand a better chance of inhibiting or blocking the activity of the natural substrate at the enzyme site. 01:36 But, there will still be a competition for that site with the substrate. Let’s have a look and see what that means in more detail. The enzyme itself will effectively be prevented from working when the concentration of the inhibitor is greater than the concentration of the substrate. 01:53 And as you can see from the bottom, when we have an excess of substrate, a reaction, the normal reaction of an enzyme would do whatever it would normally do can proceed unhindered. 02:02 In the presence of an excess of inhibitor, however, the reaction does not proceed or rather the rate of the reaction is substantially reduced because most of the active sites of the enzyme are blocked. 02:16 At lower concentrations of inhibitors, some enzyme molecules will be inhibited and some will be binding the substrate and the enzyme activity will, therefore, be reduced. Inhibitors that work in this way are described as reversible inhibitors. 02:33 Now, let’s have a look in a bit more detail about the alternative. These are also called irreversible inhibitors. Some inhibitors will bind to an enzyme permanently by making a covalent bond. 02:46 And if you go back to what we were talking about, right at the beginning, regarding bio-chemical interactions as part of Module IV, we discussed all the possible intermolecular forces that are at work: van der Waals forces, dipole-dipole, ion-dipole and so forth. 03:02 And all of these are generally found when you are looking at the characteristics of a reversible inhibitor where the energy required to overcome those intermolecular interactions is not so great. 03:14 But, when we are talking about irreversible inhibition, what we are effectively talking about is covalent bonding. When we are talking about covalent bonding, we are forming very strong bonds between the enzyme and whichever our drug happens to be and whatever our inhibitor happens to be. 03:31 And it’s often very difficult to remove them once they are in place. The inhibitor inhibition cannot be overcome by increasing the concentration of the natural substrate, in this case, because as the name suggests, the active site is irreversibly blocked. 03:47 And there are couple of good examples of this, as we will see a little later on. There is the example of aspirin which covalently binds to cycloxygenase-1. There is also, for example, organophosphates which bind irreversibly to the acetylcholine esterase active site. 04:05 Indeed, in that scenario, what happens is the actual three dimensional structure of the enzyme fundamentally changes within about half an hour, therefore, stopping the reversal of that particular inhibition. 04:20 When there is irreversible inhibition, often times what is necessary is that the enzyme itself isn’t broken down by the host’s natural systems and then reconstituted again. This takes time. 04:33 Generally, irreversible inhibition comes on quickly as soon as the concentration of the inhibitor near the enzyme rises. Irreversible inhibition may take time to come on as a... 04:46 may take time for the covalent bonds to form. 04:50 So, now, we have talked about reversible and irreversible. Let’s look at competitive and non-competitive inhibition. So, the example of reversible inhibition, described previously, can also be described as a type of competitive inhibition; competitive because, of course, we are competing with the substrate itself. The substrate and inhibitor molecules ultimately compete for the same binding site. 05:14 The example of irreversible inhibition is an example of a non-competitive inhibition since once the inhibitor binds, it stays put and the substrate itself cannot then compete to bind. 05:28 The inhibitor studied so far talk about binding in the active site and this is where you get the competition or where you have bounded selectively and irreversibly where you see a lack of competition. 05:40 But now, I’d like to talk to you a little bit about something called allosteric inhibition. 05:45 So, where you have got binding, which doesn’t take place at the active site, then maybe at a site a distance away from it, these binding sites can be known as allosteric sites. 05:55 Inhibitors which bind at these allosteric sites ultimately change the shape of the enzyme, but don’t directly influence the active site itself. However, as we will see, this can ultimately prevent substrates binding at the active site.
About the Lecture
The lecture Enzyme Inhibitors by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Which of the following statements about enzyme inhibitors is NOT true?
- Enzyme inhibitors inactivate an enzyme partially or entirely without interacting with it.
- An inhibitor molecule targeting a faulty or pathogen associated enzyme can act as a drug to cure enzyme malfunction disorder or microbial disease.
- Due to their geometrical resemblance to the substrate, competitive inhibitors are capable of blocking the active sites of an enzyme molecule.
- Reversible inhibition involves weak intermolecular forces like van der Waal’s forces, while irreversible inhibition involves covalent bonding between enzyme and inhibitor molecules.
- Irreversible inhibitors completely block the enzymatic activities of an enzyme molecule by changing the shape of the enzyme molecule.
In case of a reversible inhibition, to inhibit an enzyme to a greater extent, what should be done?
- The concentration of inhibitor molecules should be increased.
- The concentration of substrate molecules should be increased.
- The concentration of inhibitor molecules should be decreased.
- The number of enzyme molecules should be increased.
- The number of cofactor molecules should be increased.
What will happen if the substrate concentration is increased around an irreversibly inhibited enzyme?
- The enzyme will remain in an inhibited state.
- The substrate will compete with the inhibitor for the active site.
- The substrate will combine with the inhibitor molecule and change its configuration.
- An enzyme-inhibitor-substrate complex will form, and finally, this complex will dissociate into the free enzyme, free inhibitor, and product molecules.
- The enzyme will start dissociating from the inhibitor molecule.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |