Playlist
Show Playlist
Hide Playlist
Covalent Modification – Metabolism and Regulation
-
14 Basic PrinciplesOfMetabolism2.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
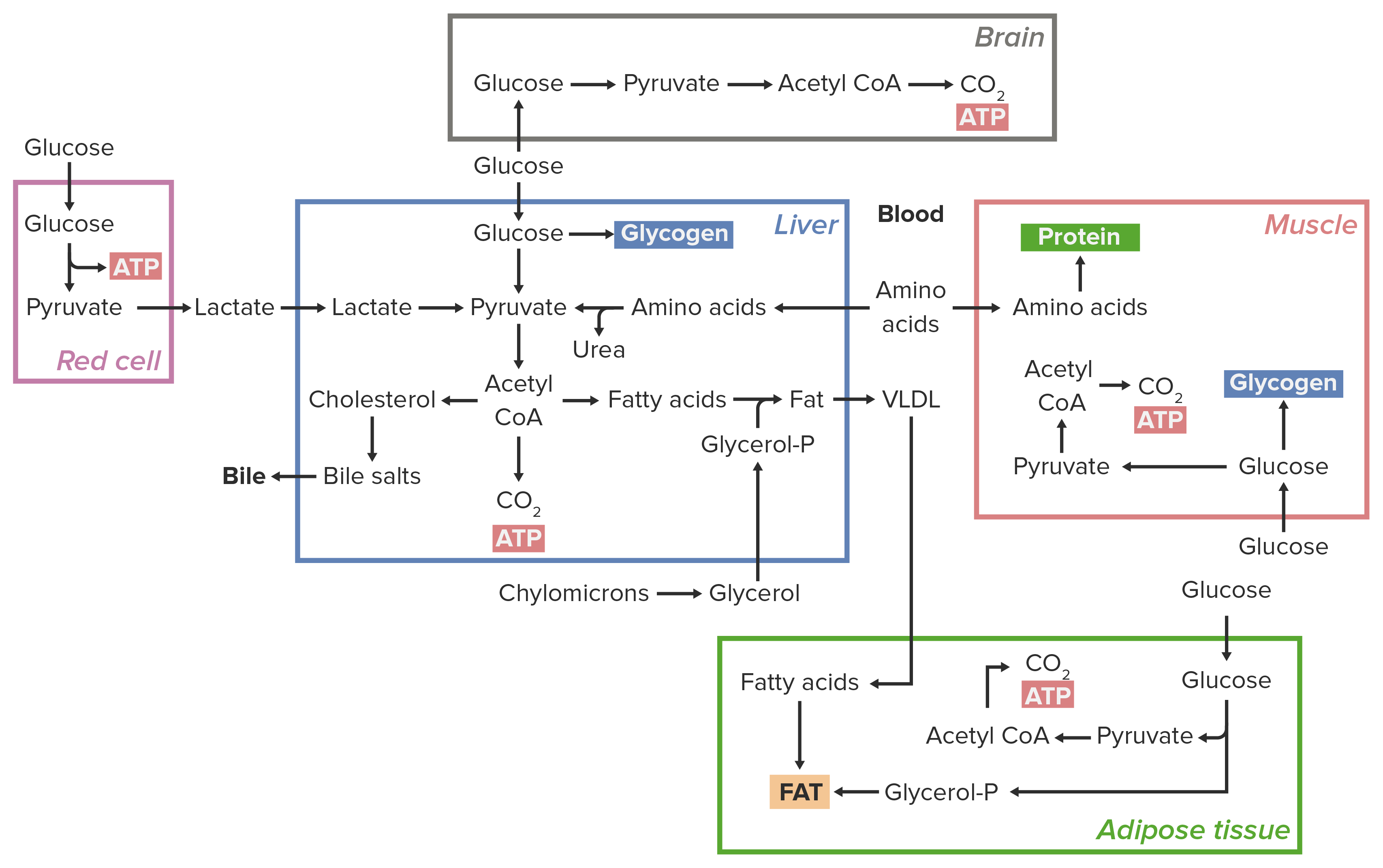
00:00 Kovalente Modifikation kann zur Kontrolle von Enzymen verwendet werden und das Beispiel, über das ich hier sprechen möchte, ist die Kontrolle der Enzyme, die am Glykogenstoffwechsel beteiligt sind. 00:09 Glykogen kann an der gleichen Stelle in einer Zelle auf- und abgebaut werden. 00:14 Es ist also sehr wichtig, dass die Zellen nur Glykogen herstellen oder nur Glykogen abbauen und die kovalente Modifikation der Enzyme bietet eine Möglichkeit, dies zu erreichen. 00:26 Wir beginnen diesen Prozess, indem wir uns auf dieser Folie die Proteinkinase A ansehen. 00:31 Die Proteinkinase A ist ein Enzym, das andere Proteine phosphoryliert. 00:35 Wenn es aktiv ist, wie in der roten Form gezeigt, setzt es Phosphate auf andere Moleküle und wie Sie hier sehen können, ist eines der Moleküle die Proteinkinase A. 00:44 Das Enzym, das dies umsetzt, ist als Phosphorylase-Kinase bekannt. 00:48 Das wandelt es von der inaktiven oder unphosphorylierten Form auf der blauen Seite zur phosphorylierten und aktiven Form auf der roten Seite um. 00:56 Die Phosphorylase-Kinase kann ihrerseits phosphoryliert werden und ein weiteres wichtiges Enzym in diesem Prozess ist die Glykogenphosphorylase B. 01:04 Die Glykogenphosphorylase B ist eine relativ inaktive Form des Enzyms. 01:08 Auch hier enthält es kein Phosphat und die Phosphorylierung produziert die als Glykogenphosphorylase A bekannte Form. 01:15 Dies ist die aktivere Form des Enzyms und wie Sie auf der Folie sehen können, katalysiert es den Abbau von Glykogen zur Herstellung von Glukose-1-Phosphat, das leicht zur Herstellung von Glukose verwendet werden kann, die wiederum für die Zellenergie wichtig ist. 01:30 Die wechselseitige Regulierung oder Kontrolle dieses Prozesses wird durch den virtuellen Effekt erreicht, dass die Enzyme, die Glykogen herstellen, durch die Phosphorylierung inaktiviert werden und die Enzyme aktiviert, die das Glykogen abbauen. 01:46 Sie können es auf dieser Folie sehen, weil die Proteinkinase A, nicht nur die Phosphorylase-Kinase, sondern auch die Glykogensynthase phosphoryliert. 01:56 Die Glykogensynthase ist das Enzym, das für die Herstellung von Glykogen notwendig ist. 02:01 Die aktive Form der Glykogensynthase ist dephosphoryliert. Sie enthält kein Phosphat. 02:08 Wenn die Proteinkinase A aktiviert wird, um Glykogen abzubauen oder den Abbau von Glykogen zu stimulieren, phosphoryliert es auch die Glykogensynthase, wodurch sie inaktiv wird und damit gleichzeitig die Glykogensynthese stoppt, da es den Abbau von Glykogen stimuliert. 02:28 Dieses Phänomen ist als reziproke Regulierung bekannt und es ist ein sehr wichtiges Prinzip für das Verständnis der Kontrolle von Stoffwechselwegen. 02:38 Bei der gegenseitigen Regulierung hat gleiche Aktion entgegengesetzte Auswirkungen auf den katabolen und anabolen Prozess. 02:47 Der katabole Prozess ist hier der Glykogenabbau, der anabole Prozess ist die Glykogensynthese. 02:52 Die Phosphorylierung der jeweiligen Enzyme hat entgegengesetzte Auswirkungen auf diese beiden Prozesse.
About the Lecture
The lecture Covalent Modification – Metabolism and Regulation by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following is true of reciprocal regulation?
- It has opposite effects on catabolic and anabolic pathways.
- It is the same as feedback inhibition.
- It always involves ATP, ADP, or AMP.
- It reverses the direction of all enzymatic reactions in a pathway.
- It up-regulates the production of the enzymes on the pathway that is being inhibited.
Which of the following enzyme gets deactivated by the protein kinase A enzyme?
- Glycogen synthase
- Phosphorylase kinase
- Glycogen phosphorylase
- Glycogen branching enzyme
- Mutase
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |