Playlist
Show Playlist
Hide Playlist
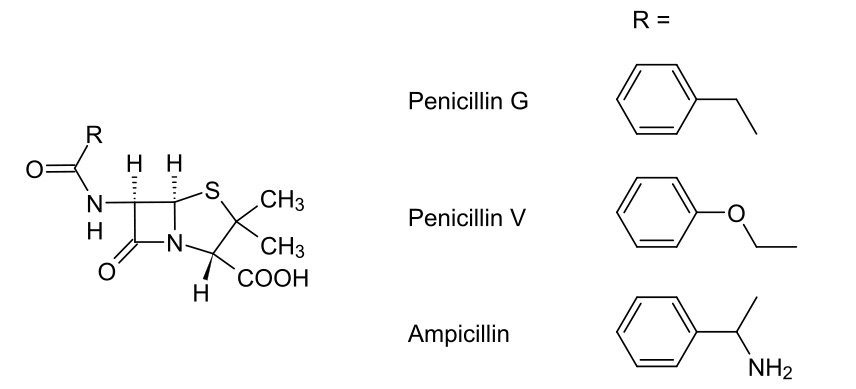
Carbapenems — Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
00:01 Now, I’d like to bring you onto the final slide for this lecture series which deals with carbapenems. 00:09 Here we have the... a model structure of a carbapenem. You should be able to identify some of the core components being very similar to the penicillins we’ve discussed thus far. 00:18 We have the beta-lactam ring, the core component required for inhibition of the penicillin-binding protein. We also have a five-membered ring which has increased ring strain. This is imparted by the addition of a double bond which forces the stereochemistry to be less thermodynamically stable. But, crucially, I’d like to draw your attention to the inverted stereochemistry. 00:42 Carbapenems have the broadest spectrum of all of the beta-lactam antibiotics, working on Gram-negative bacterial infections and Gram-positive bacterial infections and are often the last line of defence for a systemic infection in the context of beta-lactam antibiotics. 01:01 But, where they’re particularly useful is their resistance to lactamase activity. So, whereas we before had the problem where we have penicillin-resistant bacteria because they produce a beta-lactamase that breaks open that lactam ring rendering the penicillin antibiotic null and void, here we have a class of beta-lactam which, by virtue of the inverted stereochemistry in the sixth position, actually is resistant to the actions of lactamase. 01:38 If you recall, the original structure of penicillin showed a relative cis-orientation of the two hydrogens in alpha and beta positions on the lactam ring. Here we see, however, there is a relative trans-orientation on those hydrogens with, in this case, our substituted methyl group pushing backwards rather than forwards. And this is what conveys that resistance to lactamases produced by the bacteria and makes this a really, really effective beta-lactam antibiotic, working with strains that are often quite resistant to a treatment with other penicillins. This works particularly well. 02:22 However, what we are now seeing, and this is something to bear in mind, is the rise of bacteria which produce an enzyme called carbapenemase. This enzyme carbapenemase can actually selectively break down the beta-lactam ring in a carbapenem. And this is cause for increasing concern since, if we are unable to actually treat infections and so forth with carbapenem because of the rise of carbapenemase-producing bacteria, then even routine operations could become quite dangerous. 02:58 I’d like to thank you for bearing with me throughout the course of this module and also for your attention throughout this course. 03:07 Thank you very much.
About the Lecture
The lecture Carbapenems — Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Which of the following is NOT true regarding carbapenems?
- Carbapenems are highly resistant to carbapenemase attacks.
- These are the last line of defense in case of systemic infections.
- These are broad spectrum antibiotics against gram-negative as well as gram-positive bacteria.
- These compounds contain a beta-lactam ring and a five-membered ring in their general structure.
- These molecules are resistant to beta-lactamase enzymes due to inverted stereochemistry at the sixth position.
A carbapenem molecule has which of the following?
- Two hydrogen atoms with trans configuration in alpha and beta positions of the lactam ring.
- Two hydrogen atoms with cis configuration in alpha and beta positions of the lactam ring.
- Two hydrogen atoms with cis configuration in alpha and beta positions of the R1 side chain.
- Two hydrogen atoms with cis configuration in alpha and beta positions of the thiazolidine ring.
- Two hydrogen atoms with trans configuration in alpha and beta positions of the thiazolidine ring.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |