Playlist
Show Playlist
Hide Playlist
Approach to Acid Base Status: Step 3 (cont'd) – Laboratory Diagnostics
-
Slides DiagnosticsAcidosisAlkidosisStep3-5 RespiratoryPathology.pdf
-
Reference List Pathology.pdf
-
Download Lecture Overview
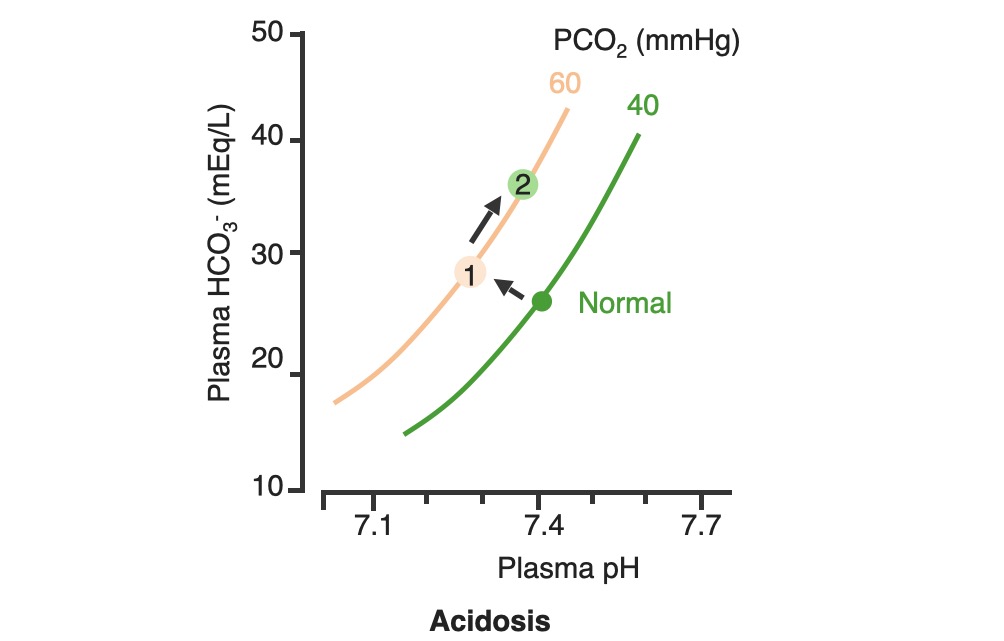
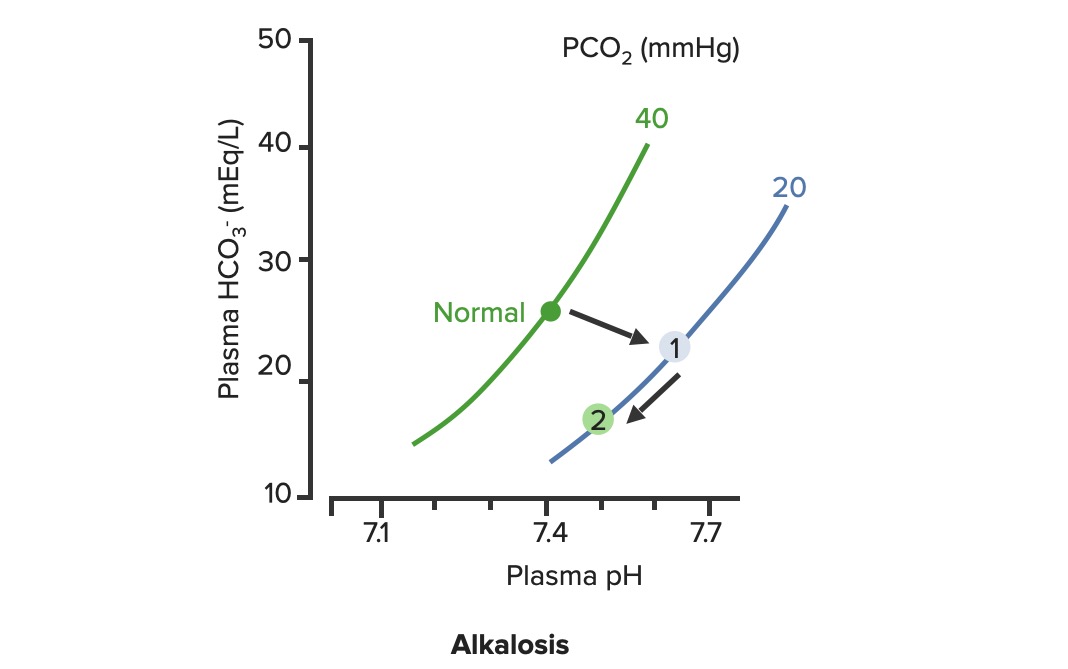
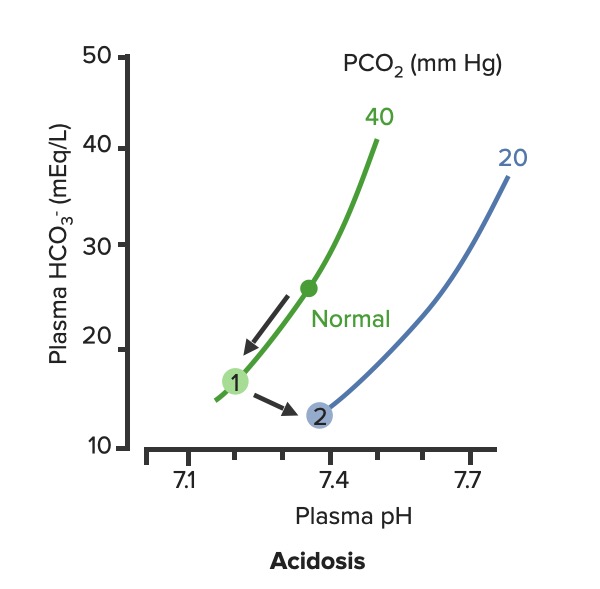
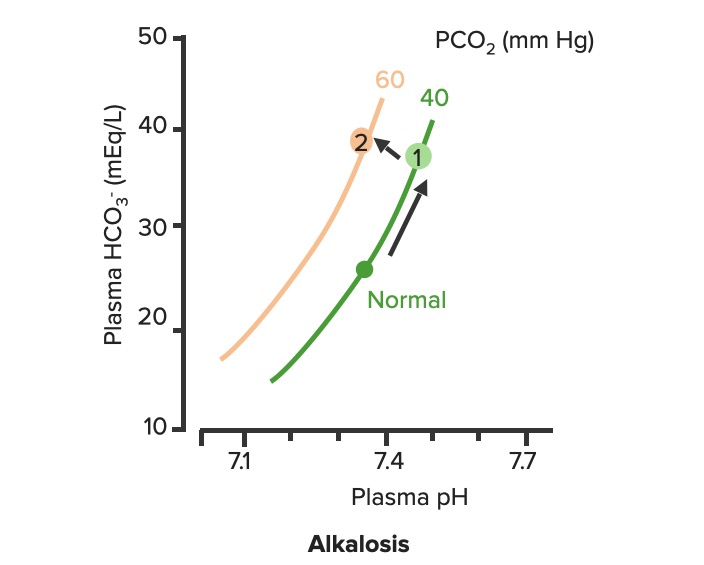
00:01 To continue our discussion with step three, in the previous discussion we looked at primary metabolic processes or pH acid base disturbance and there we start respiratory compensation, how long did it take? Minutes -- respiratory compensation. 00:15 What if you add a primary respiratory process and therefore, how are you then going to compensate with the kidney? Kidney. 00:24 Now, how long does it take for the kidney to kick in? Days not weeks, the reason I talk about weeks is because in physiology we look at high altitude, with high altitude you saw acute or chronic. 00:36 Acutely, it’s a compensation that you’re looking for so that you can then adapt to the pH imbalance. 00:45 In other words you are adapting to the respiratory alkalosis. 00:47 If it’s chronic, it means weeks. When you’re thinking about levels of what? EPO. 00:54 Remember that in high altitude, my goodness, it’s difficult to come across proper oxygenation so therefore hypoxia, therefore kidney kicks in weeks EPO increases. 01:06 What do you think EPO levels or their report in levels are in a patient that lives in high altitude? High, it has to be, but that’s weeks. 01:14 So here, under renal compensation for a primary respiratory disorder is going to be days, thus you divide into acute and chronic, acute and chronic. 01:24 For example, let’s do respiratory acidosis. 01:29 For respiratory acidosis, take me step by step here -- pH decreased, number 1. 01:34 Number 2, you time look for primary. 01:36 You find that your carbon dioxide are elevated then you know that you have a primary respiratory acidosis. 01:42 We can confirm this by how? Well, there’s some issue or differential in your patient that he or she is suffering from hypoventilation. 01:50 I’ve used opioids, let’s do another one. 01:52 Let’s say that your back looks like this and it’s curved in and you kinda have maybe a pigeon breast perhaps of your sternum, why, you might be thinking about kyphoscoliosis where literally the chest cavity cannot expand, that’s a problem, isn’t it? And so my point is this, say that you cannot breathe properly then you end up developing or increasing supplies of carbon dioxide. 02:15 Welcome to respiratory acidosis, now, with my only method of compensation, pay attention, this is where things get tricky just like it did with the degree of compensation for metabolic. 02:26 Here, in acute and chronic, we had no such acute and chronic option for metabolic and respiratory compensation, why? If it was metabolic, respiratory, immediate compensation -- there's no acute and chronic, it’s all one compensation under respiratory, there’re two, acute and chronic, why? Listen, respiratory acidosis for every 10 mmHg increase in PCO2. What’s your primary? Good respiratory acidosis, what’s my PCO2? Definitely above 40. 03:04 How’s your patient breathing? Hypoventilation, maybe opioids, I gave you a second example where literally the thoracic cage becomes constrained. 03:13 Next, acute. In acute you’ve lost a little bit of bicarb but not much, a little bit of bicarb, acutely. 03:22 You’re in the process of losing it, don’t get me wrong, so therefore if you find, let me make your life a little bit easier, if you find your carbon dioxide to be increased and your bicarb is about 23-22, what does that mean to you? Acute, doesn’t it? What if you found a carbon dioxide 40 and you find your bicarb to be 15 - what if you find your carbon dioxide to be above let’s say 40 and then what about your bicarb? Well, you find your bicarb to be increasing now, so now it’s at 24, 25, 26, 27, 28... it’s increasing. 04:02 What if your bicarb was moving in the other direction -- 23, 22, 21, 20, 18 and such? That’s a mixed disorder, so listen to what I just said there, make sure that you're clear about what’s known as partial, and then in terms of chronic and then what it means to be mixed. 04:20 Okay, so let’s talk about acute here. Acute, a pH decrease by 0.08 which then acutely means a bicarb increase by 1, both are moving the same direction, increasing carbon dioxide and you should find an increase in bicarb. 04:38 But in acute, it’s only increasing about 1 when your pH decrease by 0.08. 04:44 Versus chronic. In chronic, your pH decrease by 0.03 and your carbon dioxide increase and your bicarb here is increasing by 4, meaning to say that your kidneys are holding on, holding on, holding on, or should I say, really, that the renal threshold for reclaiming your bicarb has increased dramatically. 05:05 This is an algorithm and in the next topic or so, I’ll show you as to how to put all this together once again in a graph form. 05:13 Let us move on to respiratory alkalosis. 05:15 In respiratory alkalosis, well, this once again put you in the realm of high altitude. 05:20 With high altitude you are breathing very quickly and so therefore you have respiratory alkalosis. 05:25 Here once again you have two options -- acute or chronic. 05:28 You expect your carbon dioxide to be decrease for every 10 mm decrease in PCo2, if it’s in acute. 05:35 Now, before we move on, what do you expect your bicarb to do? You expect it to decrease. 05:39 So here once again I’ll walk you through the same. 05:41 What if you find your carbon dioxide to be less than 40 and you find your bicarb to be at 23? This is acute, it just begun the process, it’s not compensated yet; and at some point, let’s say that you find your PCO2 to be less than 40 and you find your bicarb to be 18, 17, 16... 06:02 but moving in the same direction that the partial compensation at that point. 06:06 Remember, you never get to full compensation. 06:10 And then finally, what if you find your carbon dioxide decreasing - respiratory alkalosis and you find your bicarb to be increasing. 06:17 Opposite direction, mixed. We’ve covered three in both of the branches. 06:21 Let’s talk about acute. If it’s acute respiratory alkalosis, you know that you should be dropping your bicarb. 06:28 The pH increases by 0.08, your bicarb decreases by 2, decrease, two, is what you want to memorize here, for acute respiratory alkalosis, renal compensation. 06:41 If it’s chronic, once again, pH increases by 0.03. 06:46 Here you can expect your bicarb to therefore decrease by 4. 06:50 In the long run, your then gonna end up losing more bicarb and this is what you would expect to find in a patient that have something like high altitude sickness. 06:59 In high altitude acutely they are going to have respiratory alkalosis and acutely they are trying to get rid of the bicarb ASAP from the kidney, but they’re having a hard time doing so, so therefore what would you wanna give to facilitate that exit? Good, acetazolamide. 07:17 Alright, so these are degrees of compensation and how you would wanna use the math and really the concept of renal compensation of dividing acute and chronic so the bottom line is if you take a look at the bicarb on the bottom row, respiratory acidosis, you have increase, increase, acute chronic 1 in 4. If it’s respiratory alkalosis decrease 2 and 4 so both chronic adaptations will be formed. 07:48 As I said, this information might be relatively new to you or maybe unfamiliar but it would be in your best interest to get a firm handle of all these. 07:58 Speaking of which, let’s do the same thing here as we did with the previous graphs. 08:04 First step, pH. You find the pH here, one from 7.4 and it dropped down to let’s say 7.1. 08:12 For a fact you know your patient has acidosis. 08:15 Next, next step, you tell me. Close your eyes. 08:19 If it’s primary then expect your carbon dioxide to be increased. 08:23 Let’s go and take a look at carbon dioxide, the bottom graph. 08:26 On your left, that represents your carbon dioxide on your Y axis. 08:29 You notice, please, that the carbon dioxide has increased. 08:33 Your next step, you tell me about compensation. You have the acute and chronic, don’t you? With renal compensation, good. Take a look at the top, let’s do acute first. 08:43 And we have a formula, the formula here gives you something like, well, 0.43 times the PCO2, the change in carbon dioxide and you have a plus or minus 7 or 6; and so therefore here, if you do this with acute, you’ll find that the limit is 32. What does that mean? That means that the bicarb has to increase and when it does acutely, it has a cap of approximately 32. 09:09 Now if the same method is now applied to the chronic stage; in chronic you end up getting a 45, the limit is 45 -- meaning to say that from 25 to 45, the value that you got was 20 and with that 20, what did you want to do? With that 20 did you wanna then add it to your 25 or did you wanna subtract it from your 25? Well, if you know that it’s respiratory acidosis, carbon dioxide is increased, you add it to 25 because both are moving in the same direction. 09:41 That formed us specifically there is going to be for chronic. It would be good enough to know that. 09:47 Let’s move on to the other side, same steps. The pH, well this time we find it to be increased. 09:52 We know its alkalosis, what do you expect are gonna move quicker here, what about your carbon dioxide? Well, then you expect it to decrease. 09:59 Take a look at the bottom graph and you’ll find that normally, between 35 and 45, you’ll find your carbon dioxide drop down to 20, that’s quite low, 20. 10:08 And if you expect your carbon dioxide decreased then yeah, once again, acute and chronic, acute and chronic. 10:14 So here, you find it acutely, you find a change and the limit here was 18 or if it is chronic, then it moved down to 12. Understand the concepts, you’ll be in good shape, don’t forget about acute and chronic and then on the previous figure with the algorithms it then showed you how much of a change in pH equates to a change and either you use 1 and 4, 2 and 4 depending as to what kind of compensation. 10:43 Spend a little bit of time here, understand the concepts, let us now move on.
About the Lecture
The lecture Approach to Acid Base Status: Step 3 (cont'd) – Laboratory Diagnostics by Carlo Raj, MD is from the course Pulmonary Diagnostics.
Included Quiz Questions
Which of the following correctly represents the process that occurs in a patient with severe kyphoscoliosis?
- Respiratory acidosis with chronically increased bicarbonate.
- Respiratory acidosis with acutely increased bicarbonate.
- Respiratory alkalosis with chronically increased bicarbonate.
- Respiratory alkalosis with acutely increased bicarbonate.
- Metabolic acidosis with chronically decreased bicarbonate.
Which of the following describes the lab values in chronic respiratory alkalosis?
- Increased pH, decreased PCO2, and decreased bicarbonate.
- Decreased pH, decreased PCO2, and decreased bicarbonate.
- Increased pH, decreased PCO2, and increased bicarbonate.
- Increased pH, increased PCO2, and decreased bicarbonate.
- Increased pH, increased PCO2, and increased bicarbonate.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |