Playlist
Show Playlist
Hide Playlist
Anticoagulation Therapy
-
Slides Hemostasis Therapies.pdf
-
Reference List Pathology.pdf
-
Download Lecture Overview
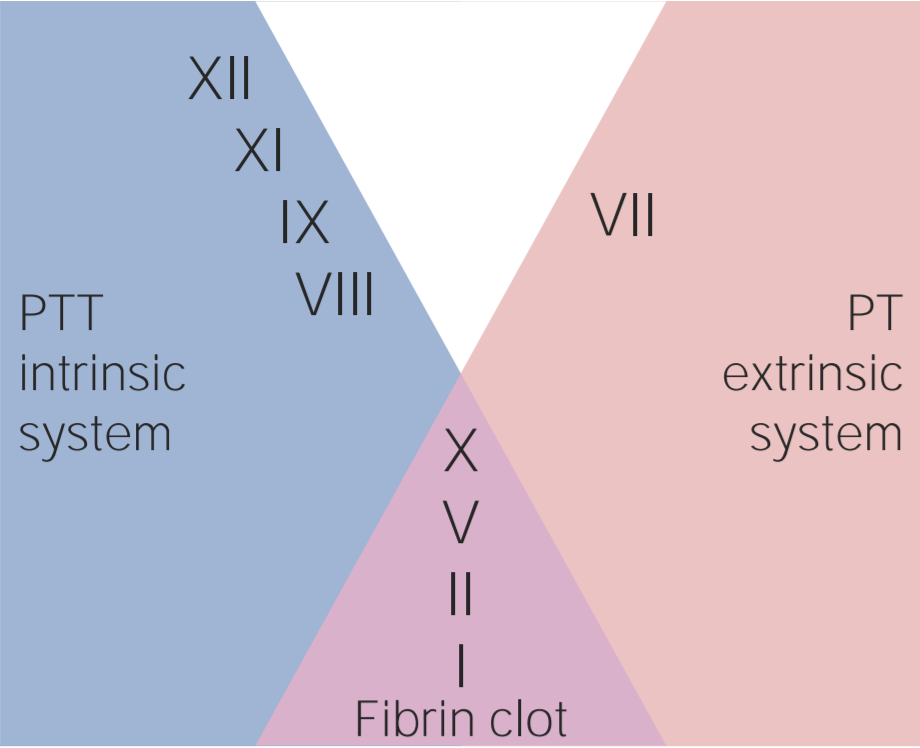
00:02 We've come a long way. 00:03 We've talked about, normal hemostasis. 00:06 We've talked about, pro and anticoagulant factors. 00:09 We've talked about, how to bring platelets into the equation and the important role of endothelial cells. 00:15 We've talked about, how to measure the various coagulation factors and we've talked about, bleeding and thrombotic disorders. 00:25 Finally, we're going to talk about, therapies. 00:27 So, ultimately, it's important to know all of this, that's come before, because, it very much informs, how we treat patients who either, too coagulant or not coagulant enough. 00:41 In other words, they're clotting inappropriately or they're bleeding inappropriately and knowing, how to, modulate the coagulation cascade, how to affect platelet function, is going to be a major weapon in your armamentarium, when you're treating patients, so really important to know. 01:00 Therapies, so, this is where we've come all the way through down our road map and we're getting now into the final step, so, hang in there, we're almost done. 01:12 Oh my goodness, we're back to the intrinsic and extrinsic pathway, having to do with coagulation. 01:18 That's because, we're going to be talking about the various ways, that we can impact, this multi-step protein cascade. 01:27 Heparin, is one of the mainstays. 01:30 Heparin, as you will recall, endothelial cells make a heparin-like molecule, that interacts with antithrombin III and that affects, that regulates turns off the activity of factors XII, XI, IX, X and IIa. 01:49 We can give exogenous heparin and activate more of the antithrombin III and so inhibit the coagulation of those factors and so we do this to prevent people from thrombosing, if they're lying around in a hospital bed, you will regularly dose patients, who are relatively immobile, not getting out of bed, with, subcutaneous heparin, to drive exactly this inhibitory pathway. That's great. 02:18 There are some downsides, so about 5% of the population can develop antibodies, that form against heparin and a platelet factor. 02:29 So, this is so called the heparin induced thrombocytopenia disease. 02:35 When that happens, we go to a different form of heparin, we use, “Low molecular weight heparin,” "L-M-A-W-H." And that acts, not so much, on XII, XI, IX, X, but acts on just factor IIa thrombin. 02:54 It tends not to induce as much of that heparin-induced, thrombocytopenia or hit syndrome. 03:00 So, in many cases where there may be a risk of that, we will give that low molecular weight heparin, but it blocks very effectively the IIa. 03:11 A variety of drugs, that you will encounter, have an “x,” in the name. 03:14 So, "x" marks the spot. 03:16 Fondaparinux, rivaroxaban, apixaban, All those, act on factor X and prevent its activation. 03:26 So, this easy to remember if you see x with some rare exception. 03:30 So, that's another way that we can block the common pathway involving factor X. 03:37 We can give, argatroban, dabigatran, bivalirudin, these will specifically inhibit the activity of thrombin. 03:47 These are incredibly potent. 03:50 So, the nice thing about heparin and low molecular weight heparin, they are relatively easy to turn on and turn off. 03:57 Fondaparinux is the next level up, so, the things that activate and inactivate factor 10 next level up, somewhat more difficult to regulate and fine-tune and then when we get into the argatroban and company, acting at thrombin, those can be very, very potent anticoagulants and will can cause a lot of bleeding. 04:23 Finally, coumadin, going to be one of the mainstays, that you will use in the treatment of patients, who have a procoagulant tendency. 04:34 That's because it acts on both sides of the intrinsic and extrinsic pathway. 04:39 It will, act to prevent the development as we'll see in the next slide, of activated factors II, VII, IX, and X. 04:51 How is this happening? So factors II, VII, IX, and X, require a second carboxy group, to be added to glutamates. 05:02 So that step, converts glutamate to a carboxy glutamate, in the final protein. 05:08 By having those two carboxyl groups, side-by-side, that gives us a localized negative charge of -2, that will chelate or will interact with calcium. 05:20 So, that allows factors II, VII, IX, and X, to specifically localize to calcium in phospholipid areas, so that, we can get the appropriate activation and if we don't put the second carboxyl group on, those factors are not active. 05:34 So, how do we put the second carboxyl group on? It requires the activity of a vitamin K. 05:40 And by going from glutamate to carboxy glutamate, from left to right, that vitamin K gets inactivated. 05:47 Now, GI tract microbiome and our own intrinsic synthetic capacity, does make vitamin K, but if at an inadequate level, if we're not constantly regenerating activated vitamin K, from the inactive form and that, there is an enzyme pathway that does that. 06:05 That back activation pathway, that we need to keep vitamin K levels up to snuff, can be turned off by coumadin. 06:14 So, that's the activity of coumadin. 06:16 And the reason that it was used so effectively originally as a rat poison, is that basically, you were causing the inactivation of factors II, VII, IX, and X, because you didn't add the carboxy group and rats that ate the poison that had coumadin in it, were bleeding to death, they were not clotting appropriately. 06:38 We don't give the same levels of coumadin as we do in rat poison, to people, but we do give very low levels that allow us to coregulate the activities II, VII, IX, and X. 06:50 Okay, so that's the coagulation pathway. 06:53 The proteins and how we can impact those. 06:56 How can we affect platelets? So, recall that, ADP and there are other molecules as well, but ADP, primarily through a receptor, the ADP receptor, on the surface of platelets, will drive a variety of pathways within that platelet. 07:11 Along the top line, we will get IIb/IIIa activation, so that we can get platelet to platelet interaction through fibrinogen. 07:18 We will drive the metabolic breakdown of arachidonic acid, via cyclooxygenase to produce thromboxane a2, which drives greater platelet aggregation, but will also drive granule release. 07:33 So, there are a lot of things that happen once we bind ADP, “Adenosine Diphosphate.” Wow, there are lots of targets here let's start hitting them. 07:42 So, there are drugs that will block that ADP-ADP receptor interaction, clopidogrel and ticlopidine and these are very commonly used to inhibit platelet activity. 07:54 We can give aspirin or any of the other non-steroidal anti-inflammatory agents, which will, block the cyclooxygenase, which means, we don't make thromboxane A2, which means, we don't get granular release and further platelet activation. 08:07 We can also block, IIb/IIIa, this is with a variety of molecules, including a monoclonal antibody called abciximab or tirofiban and both of those, will block, that interaction linking platelet to platelet through the glycoprotein IIb/IIIa, surface molecule.
About the Lecture
The lecture Anticoagulation Therapy by Richard Mitchell, MD, PhD is from the course Hemostasis.
Included Quiz Questions
What factors are inactivated by heparin?
- IIa (thrombin), X, XII, XI, and IX
- X, IX, VIII, VII, and IIa (thrombin)
- V, VI, VII, VIII, and IIa (thrombin)
- I, IIa (thrombin), III, IV, and V
- VII, VIII, IX, XII, and IIa (thrombin)
Low-molecular-weight heparin (LMWH) acts on what factor?
- IIa (thrombin)
- XII
- XI
- IX
- X
What is the mechanism of action of warfarin?
- It blocks the regeneration of active vitamin K.
- It increases the activity of plasminogen.
- It catalyzes the breakdown of fibrin.
- It enhances the production of vitamin K.
- It produces an antithrombin activator.
What pathway is blocked by aspirin?
- Cyclooxygenase pathway
- ADP receptor interaction
- Activation of GPIIb/IIIa
- Release of platelet granules
- Platelet adhesion
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |