Playlist
Show Playlist
Hide Playlist
Amino Acids – Amines
-
Slides 09 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
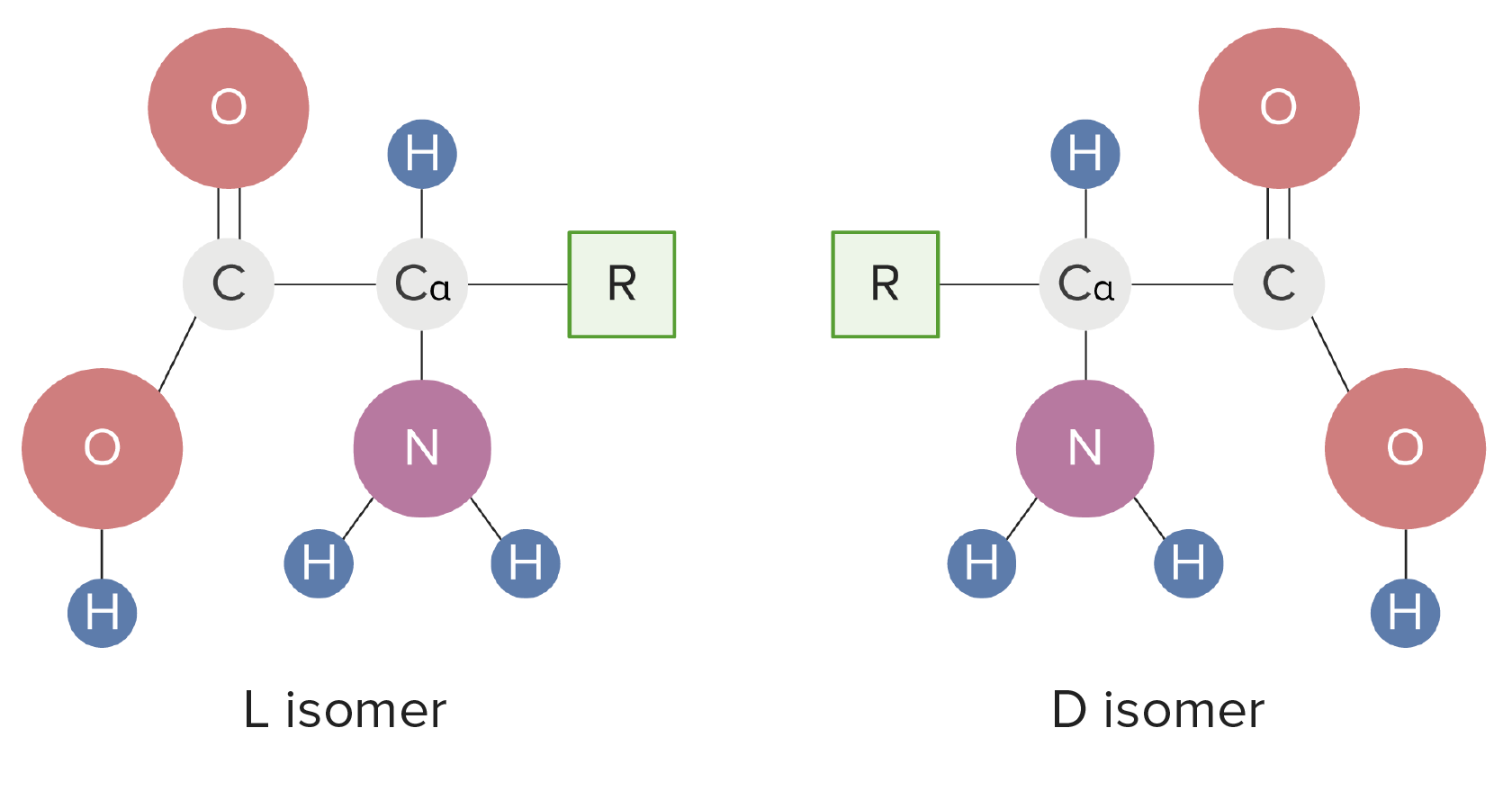
00:01 Now, let us move on to amino acids because these are very important species from a biological perspective. Amino acids derive their name from a combination of the amine group and the carboxylic acid group to give a so-called amino acid. Specifically, at the chiral center, which, in this case, is sinister, it is called an alpha amino acid, alpha being a way of explaining or a way of defining a carbon which is one carbon away from a priority group, which in this case, is most likely to be the carboxylic acid. 00:34 In this scenario, all amino acids which are naturally occurring are L, okay, or levorotationary. 00:40 Now, I have to say there are maybe a couple of exceptions to this rule in terms of chemical messengers, one of these being N-methyl-D-aspartate, an amino acid derivative. But, by and large, the ones you will come across and the ones which are found in majority of proteins are going to be levorotationary. So, let’s have a look at amino acids in a bit more detail. And this is important to understand because this influences, in terms of protein therapy and therapy with polypeptide-based drugs, how well, indeed, they’re absorbed. 01:17 If we look at alpha amino acid in its own right and we consider it in its free form, we find that it is actually in dynamic equilibrium with its so-called dipolar structure or zwitterionic form. Hopefully, you can appreciate that when we have an acid component and a basic component on the same molecule, it’s possible for the electron pair from the nitrogen to be nodated onto the hydrogen of the carboxylic acid and actually deprotonate it, thus resulting, as you can see here, in the carboxylic acid carboxylate conjugate base, COO-, and the ammonium, NH3+. 01:52 Dipolar ions are typically found at 7,38 pH. pI is another measure which correlates to the isoelectric point and this is the pH at which the amino acid is actually found in the zwitterionic form and it varies from amino acid to amino acid. 02:16 So, here we have three possible conditions or three possible structures for our amino acid. In the sense that we have this zwitterionic form, where we have protonated NH2 to give us NH3+ and the deprotonated carboxylic acid to give us the conjugate base of the carboxylate. 02:36 At pH 1, which is where we have the most acid, so in other words, we have the most H+ kicking around, both the carboxylic acid and also the amine are protonated. This gives us the carboxylic acid on its own and not the carboxylate and this gives us the NH3+, ammonium. 02:55 Around pH 7, we see we have the zwitterionic form, as we said before, where we have protonation of the NH2 and we have the carboxylic… carboxylate salt. And here, at pH 11, which is a basic pH, that there is insufficient concentration of H+ to protonate the NH2 and we have, by virtue of the amount of hydroxide present, deprotonated the carboxylic acid to give us the conjugate base. Importance in amino acids. 03:28 Well, amino acids are the building blocks of peptides and proteins and proteins are, you know, the basis on which key chemical reactions can and must occur in order for life to exist. They play a crucial role in practically every biological process and there are 20 naturally occurring amino acids. I’ve shown here an example of a hemoglobin protein, which is essential for transporting oxygen and carbon dioxide to and from. 03:55 Amino acids which can’t be synthesised in the body fall into these… into this number here: lysine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine and arginine. These cannot be synthesised and are actually required by diet and this is usually in the form of meat and dairy products. However, if meat and dairy is not consumed, they can be supplied by a combination of cereal grains such as wheat, corn and rice or legumes, beans and peanuts. Of course, amino acids, as individual molecules,
About the Lecture
The lecture Amino Acids – Amines by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Which of the following statements about amino acids is FALSE?
- Essential amino acids are dextrorotatory in nature.
- There are 20 naturally occurring amino acids.
- Amino acids are building blocks of proteins.
- All naturally occurring amino acids are levorotatory.
- Amino acid molecules have both amine and carboxylic groups attached to the same carbon atom.
Which of the following statements about zwitterions is FALSE?
- All amino acids exist in zwitterion form at pH 7.38.
- A dipolar ion or a zwitterion is a natural molecule with separate negative and positive charges on the same amino acid.
- Zwitterions of amino acids contain a carboxylate and an ammonium group due to the amino acid intramolecular acid-base reaction.
- Zwitterions of amino acids are more soluble in water than amino acid molecules due to favorable electrostatic interactions with the surrounding water molecules.
- pI or isoelectric point of each amino acid varies from amino acid to amino acid.
The isoelectric point of an amino acid is which of the following?
- The pH at which an amino acid does not move in an electric field and exists in zwitterion form.
- The pH at which an amino acid does not exist in zwitterion form.
- The pH at which an amino acid moves towards the anode under the influence of an electric field.
- The pH at which an amino acid exists as a conjugate base of carboxylic acid.
- The pH at which an amino acid migrates towards the cathode in an electric field.
Which of the following statements about essential amino acids is not true?
- The human body is capable of synthesizing essential amino acids at elevated body temperatures.
- The ten essential amino acids are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
- Lack of essential amino acids in the human diet leads to a medical condition or body malfunctioning.
- Essential amino acids must be incorporated in the diet to prevent amino acid deficiency disorders.
- The human body cannot synthesize the essential amino acids.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |