Playlist
Show Playlist
Hide Playlist
Acute Leukemia – Leukemia
-
Slides Leukaemia.pdf
-
Reference List Hematology.pdf
-
Download Lecture Overview
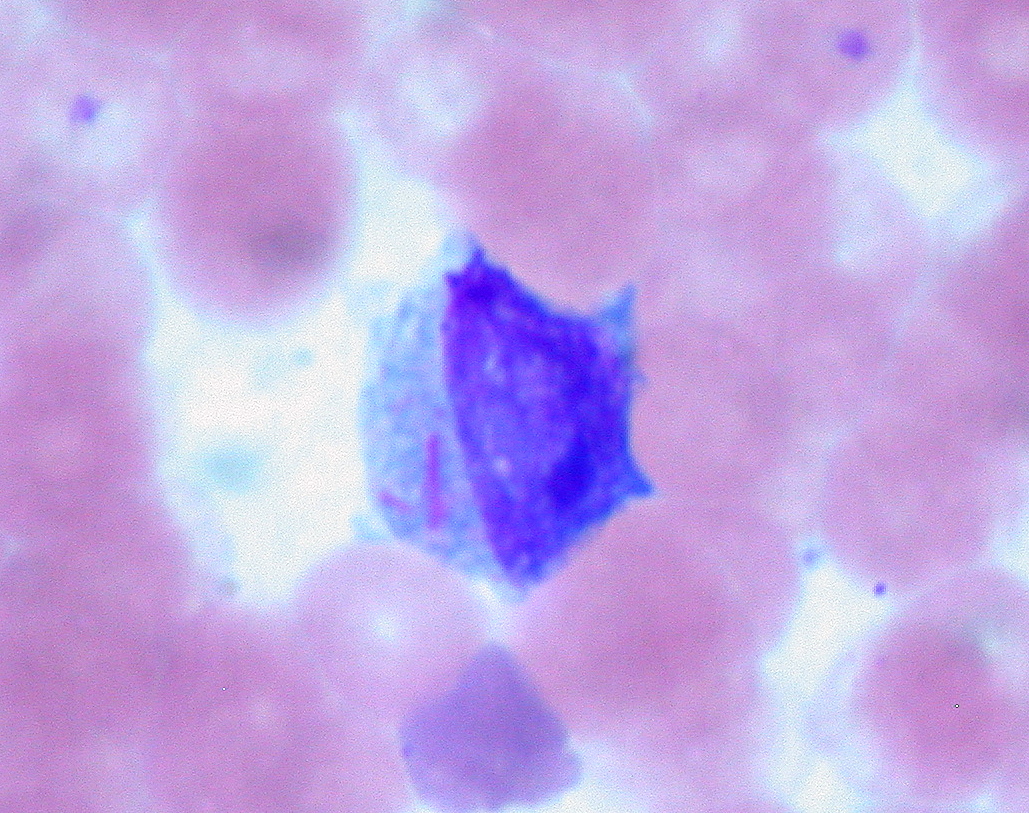
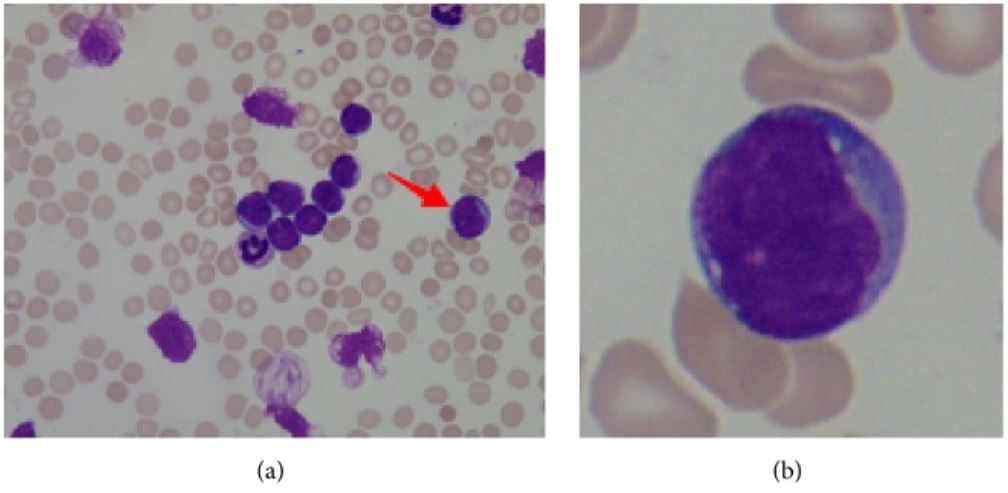
00:00 Let me now focus on each of these leukaemias individually and we will start with acute lymphoblastic leukaemia. This is the most common type of malignant disease in children, but can also develop in adults. If you look on the right-hand side here, we see in the incidence rate per 100,000 population according to the age at diagnosis on the X-axis and you will see the peak in much younger children, which we will also see that all populations at any age have a risk of developing acute lymphoblastic leukaemia and, fortunately, the older the one gets, the more challenging it is to achieve a cure for this disease. Now if you look at the left-hand side of the slide and work through those points, she will see that in children it is strongly believed now that the first mutation causing this leukaemia starts in utero before the child is even being born. This is fascinating for a number of reasons because it means we have to be very cautious and thoughtful about the health of a pregnant woman with risk factors that she may be exposed to before even childbirth. How on earth do we know this? How is this conclusion arisen? Occasionally identical twins are born and one of the twins may develop acute lymphoblastic leukaemia and, unfortunately, the second twin also has an increased risk and when scientists have looked at leukaemia that develops within these twins, they can see that they have developed from the same cell that was present before the twins were born and were shed in the placental circulation and so that less from rare cases of identical twins is now thought really to apply to all cases, but it needs more the born mutation for the tumor to develop and so something during early childhood leads to more genetic damage and the development of the . . . leukaemia. When leukaemia develops, it tends to present with swollen lymph nodes within the neck perhaps in the groin and also the clinical features of bone marrow failure and I want to emphasize the bone marrow failure is the clinical feature of all acute leukaemia. What does it mean? Well, it means that because the acute leukaemia cells crowd out the normal bone marrow function, normal blood cells are not made that means the patient get anaemic like red cells so they are tired and lethargic. It means that they are not making white cells so that prone to infections and finally not making platelets and can have bruising or bleeding. A typical feature is small bruises on the legs so called purpura and that is often seen in children who present with acute leukaemia. Now the diagnosis of the acute lymphoblastic leukaemia is made by a number of different tests obviously history, examination with the blood film, immunophenotyping to define the protein expression on the tumor cells and also very importantly these days genetic analysis of the tumor cell to see which specific genes become damaged. Here we have got some of the typical genetic changes that you see in acute lymphoblastic leukaemia. There is too many for us to work through during the lecture, but I will pick up some of the most important features and on the right you can see the prognostic significance so as well as the type of genes and chromosomes that are damaged in defining acute lymphoblastic leukaemia. We can also give a prognosis that patient as to their likely outcome. So if you look down on the left-hand side of that table words says abnormality, let us look first at numerical change. This is a change in the number of chromosomes within the tumor cell. So remember that all of the cells in our body perhaps 10 to the power of 14 or 10 to the power of 15 cells in your body, they have all got 23 pairs of chromosomes. But if the number of chromosomes in a cell increases that is called hyperdiploiding and you will see that if a tumor cell in ALL acute lymphoblastic leukaemia has high hyperdiploidy well over 50 chromosomes that are a good feature and most patients do well whereas if we go down slightly to hypodiploid, a reduction in a number of chromosomes, that is a poor prognostic sign. So just counting the number of chromosomes within a tumour cell already gives you some prognostic information to the outcome of leukaemia. But the other feature about the genetic is the structural abnormality, which specific genes are affected in the tumor and also which translocations are present. 05:49 They are also very important in prognosis. Now I introduced to new word there translocation. 05:59 What is that? What it is as we will see later with chronic myeloid leukaemia, the classic example of the translocation. Two chromosomes break and they join together. You will see an example that the Philadelphia chromosome, which brings together chromosomes 9, 10, 22 to make the BCR-ABL protein. That is definitive of chronic myeloid leukaemia but is also seen in some patients with acute lymphoblastic leukaemia and as you will see that is the poor prognostic sign in acute lymphoblastic leukaemia. But just below that you will see the t(12:21) the joining of chromosomes 12 and 21 and the genes there are the TEL-AML1 genes coming together. That is actually a good prognosis and you will see the patients with that translocation can look forward to a better outcome after treatment. So you can see how we are starting to get more and more information about the types of genes that lead to acute lymphoblastic leukaemia and how they define the clinical outcome. 07:17 The treatment of acute lymphoblastic leukaemia is getting better but it is really very complex and it involved blocks of chemotherapy given for even 2 or 3 years. The initial chemotherapy is very important and it must be given quite quickly. It seeks to kill off the great majority of tumor cells within the patient and achieve a normal blood count. We call this remission induction, we have induced a remission in the patient. The blood count is recovered, this is very effective in most people with acute leukaemia. The drugs that we have can often achieve a remission, but that is not enough to kill the patient because there are many many tumor cells remaining within the body and therefore we have to move to further causes of intensive chemotherapy to remove more and more of the remaining tumor cells. 08:23 We call this consolidation therapy and it is given as intensive blocks of combination chemotherapy. 08:31 Finally less intensive treatment in the form of tablets or injections can be given for 1 to 2 years as a maintenance therapy quite an unusual form of cancer therapy but one that is proving to be very effective in acute lymphoblastic leukaemia. Now the types of drugs that we use for treating this disease are really very diverse and are interesting to spend a few minutes talking about the nature of these drugs and you will see just a few examples here. Steroids which, of course, are used very widely in medicine and a range of indications are very effective and they are very good at killing of lymphocytes, lLymphoblasts and, of course, all the tumor cell within this disease. That is probably why steroids are so good for treating autoimmune conditions and inflammation through this activity. Vincristine is a very important drug derived from plant material, which affects the microtubules within a cell and is a very good drug against lymphoid diseases. Unfortunately, it can be quite toxic to nerves and one of the problems with Vincristine is that sometimes patients get numbness or tingling within peripheral nerves. Daunorubicin is an important chemotherapy agent, which acts to stop DNA being able to be underwhelmed and replicate effectively. Asparaginase is a very interesting drug because it is a natural enzyme that depletes asparagin an aminoacid and the reason that is useful in acute lymphoblastic leukaemia is that the leukaemia cells need a lot of asparagin to continue to divide and so effectively this treatment is depriving them within the microenvironment of enough essential amino acid to reproduce the cell and divide, so a highly effective therapy. So more cells, more drugs here used an acute lymphoblastic leukaemia, Cytosine arabinoside often known as AraC. It is an analogue of cytosine, which is used as a building block for making DNA. This is a very very effective agent in acute leukaemia as you will see soon. This is the key drug used in acute myeloid leukaemia as well. Now one feature of acute lymphoblastic leukaemia that is being noted ever last 30 years is the patients often have a tendency to have a relapse of the disease within the central nervous system. It seems that these tumor cells can move to the central nervous system and needs special treatment to eradicate the disease in that environment. 11:44 There are two major approaches. One is intrathecal chemotherapy that means chemotherapy that is given into the cerebrospinal fluid directly. Another is to use high-doses of intravenous chemotherapy of drugs such as Methotrexate, which get into the central nervous system and can help to kill off the leukaemia cells. Radiotherapy of the brain and spinal cord can also be used but where possible we tried to avoid that because of potential damage to the CNS from using radiotherapy in the long term. 12:29 Now, the outcomes of acute lymphoblastic leukaemia after all its treatments have been given are really quite good. The great majority of children with acute lymphoblastic leukaemia can expect to achieve a long-term cure. There are some children do relapse after the chemotherapy, which of course is terribly disheartening after everything they have been through and what doctors will probably try to do that is to get further chemotherapy or bone marrow transplant in which patient is given chemotherapy and then stem cells are taken from the blood and bone marrow of another person matched for the right tissue type and given in the patient and bone marrow transplantation is an effective treatment for ALL although it does have side effects. If you take everybody together, around 85 percent of children can expect a long term cure through these treatments and that number is increasing every few years as new reagents and new combinations of treatment are brought in to therapy. But unfortunately in adults, the disease outcome is not as good particularly these people get older. 13:55 This is due to the type of genetic damage that we will see within the tumor cells with age and also the ability of patients to tolerate the high-dose of chemotherapy that are needed to eradicate this disease. Let us now move to the second major subtype of leukaemia, acute myeloid leukaemia. On the right, I've got a couple of slides to show you. 14:25 On the top, you will see those blast cells of acute myeloid leukemia. 14:31 Four of them together within the blood. 14:34 So, acute myeloid leukemia is seen in people of all ages. 14:40 It can just arise on its own or it may develop from pre-malignant conditions such as myelodysplasia or myeloproliferative disease. 14:54 I'll be discussing those disorders in another lecture. 14:57 The net outcome is that we see an accumulation of primitive myeloblasts. 15:04 And just as for acute lymphoid leukemia, genetic analysis is very important in classifying a subset of AML that we are dealing with. 15:16 That's seen on this slide here. So, the subtypes of AML are very, very complex. 15:26 A primary AML is one that arises as a new disease on its own. 15:32 Whereas, the disorder may be secondary to myelodysplasia or previous chemotherapy. 15:40 Perhaps, somebody who had treatment for breast cancer five or ten years ago. 15:45 And in this situation, the AML is more challenging to treat. 15:50 And just as with ALL, acute lymphoblastic leukemia, the genetic analysis will classify the AML into different risk groups. 16:01 Some patients have a good risk outlook, some are standard, and some are poor. 16:09 And as well as just being able to say for the patient or the doctor that we can predict outcome, this information is more useful in that because it can make a decision as to which treatment is used for the individual patient. 16:23 Now, the figure on the right shows how these different types of acute myeloid leukemia are seen in patients of different age. 16:35 On the X axis, we see patient age from naught to 14, to 60 and over, and on the Y axis, the percentage type of disease. 16:47 And now, we'd really only pick out two major factors from that chart. 16:55 In green, secondary AML and you will see that as patients get older, their relative frequency of secondary AML increases. That makes sense. 17:06 Patients have had more time to accumulate damage or to have previous chemotherapy. 17:13 And secondly, the type of AML associated with good risk goes down as patients get older. 17:23 So, you can see already that younger patients have a better outlook for that AML treatment. 17:30 And how are we going to treat AML? Well, again, this is a very intensive chemotherapy similar in principle to the way that we treat acute lymphoblastic leukemia. 17:46 So, that induction chemotherapy that we give for our patients after diagnosis tend to come into hospital and be given intravenous chemotherapy. 17:55 Here, it's given with AraC, cytosine arabinoside, and daunorubicin. 18:03 And that combination given for few days is really highly effective in killing off the vast majority of acute myeloid leukemia cells and then the patient can recover after two or three weeks into a normal blood count. 18:19 Now, that's reassuring and the patient is, for the short term, relatively safe at that point. 18:27 However, as with ALL, that is not the curative approach. 18:33 We need to consolidate that with several causes of intensive chemotherapy. 18:40 These are again given as intravenous drugs into the vein. 18:47 The patient may come into hospital for them or they may stay at home and just come in for daily injections. 18:55 The risk here is that the patient is rendered really quite prone to infections, not so major challenge. 19:02 So, it's a challenging treatment but at the end of it, many patients are indeed definitively cured. 19:09 Now, at the bottom there, I've mentioned stem cell transplantation and this may be used for patients with acute myeloid leukemia. 19:20 It's particularly used for people with high-risk disease because we know that chemotherapy is not very good in this situation. 19:29 And it's also very effective in patients where the disease relapses. 19:35 That means that a patient has undergone all of the chemotherapy and then perhaps, a few months or a few years later, the disease returns. 19:45 We know that in that situation, giving the same chemotherapy again is unlikely to get as a cure whereas a stem cell transplant can be highly effective in this state. 19:59 Just a few words about stem cell transplantation, it used to be known. 20:04 You may know it as bone marrow transplantation. 20:08 The name changed because we now get most of our donor stem cells from the blood of the donor, rather than having to go to the bone marrow. 20:18 And the important thing to remember about stem cell transplantation in acute leukemia is that the stem cells come from another person. 20:28 It's what we term allogeneic stem cell transplantation. 20:32 We used to do stem cells from the patients own blood and bone marrow which should be in-stored prior to delivery after chemotherapy. 20:42 That's not so effective. And the final point which is very interesting is, when we do a stem cell transplant from another person, those cells that we put in undergo a very interesting immune effect where the stem cells attack the leukemia within the patient. 21:03 We call that a graft-versus-leukemia effect and it's highly effective in mediating the cure that arises from a stem cell transplant. 21:14 Now, then. There is one subset of acute myeloid leukemia that needs to be treated very differently and that is this disorder that you can see on the slide on the right, acute promyelocytic leukemia. 21:32 This is a tumor of promyelocytes and as you'll see, they are very, very granulous cells. 21:40 Now, APML, acute promyelocytic leukemia, is caused by a specific translocation in most cases, a translocation between chromosomes 15 and 17. 21:58 And the major clinical concern of this disease within the first few days is a very high incidence of a disorder called disseminated intravascular coagulation, DIC. 22:13 Severe bleeding tendency and unfortunately, this can be fatal within the first few days of treatment or presentation before the chemotherapy has had time to work. 22:27 And so, regular clotting tests and administration of products such as fresh frozen plasma is a critical component of the therapy of patients with acute promyelocytic leukemia. 22:42 Now, there's a very interesting story about this disease. 22:45 The t(15;17) translocation involves the retinoic acid receptor. 22:52 And therefore, doctors tried all-trans retinoic acid or ATRA as a therapy, simple oral tablet, and indeed, it's a highly effective therapy which should be given immediately a patient is diagnosed with this disorder. 23:09 On its own, it doesn't seem to be curative in high number of patients so it's often combined with chemotherapy but usually, a relatively milder chemotherapy has been given for other forms of AML. 23:25 Would you believe arsenic is now also being used for the treatment of acute promyelocytic leukemia and is proving highly effective. 23:33 So, it's really a very fascinating subtype of acute myeloid leukemia. 23:37 The outcome of AML as a whole is improving but remains challenging. 23:47 The supportive care of patients with acute leukemia has been critical in achieving the good outcomes that we can now obtain. 23:57 This involves the use of antibiotics, blood product transfusions, and indwelling catheters. 24:05 You'll see on the right, a classic chest x-ray and you'll see the heart, lungs. 24:12 You may notice if you look carefully, a line there, so-called Hickman line, which is going in to the great veins of that patient and sitting into the superior vena cava. 24:25 And from that line, blood can be drawn, taken out to measure the blood count or to culture for the presence of bacteria and also used to give chemotherapy or blood products. 24:39 And you can see how much more humane this is for the treatment and how it helps to improve outlook. 24:47 The cure rates for acute myeloid leukemia are not yet at the level of ALL, acute lymphoid leukemia in children, but certainly now, we're over 50% in most cases. 25:00 But the main challenge remains in the older-aged group and the five-year survival for people over the age of 65 with acute myeloid leukemia is less than 10%, even less than 8%. 25:14 So, that's an area where we need much more scientific advance and new clinical interventions.
About the Lecture
The lecture Acute Leukemia – Leukemia by Paul Moss, PhD, OBE, FMed, FRCPath is from the course Hematologic Disorders.
Included Quiz Questions
Which of the following is a common site for relapse of acute lymphocytic leukemia?
- CNS
- Skin
- Liver
- Kidneys
- Lungs
Which one of the following drugs is NOT used in the treatment of acute lymphoblastic leukemia?
- All trans retinoic acid
- Asparaginase
- Steroids
- Vincristine
- Daunorubicin
Which of these statements is NOT TRUE regarding acute myeloid leukemia?
- Allogeneic bone marrow transplantation is only used in patients with low-risk disease
- It is caused by an inappropriate production of myeloblasts
- Genetic analysis is valuable in predicting the outcome
- It may develop from myelodysplasia
- Stem cell transplantation is more effective when the stem cells are taken from another person (not the patient)
In which age group is acute lymphoblastic leukemia commonly seen?
- 2 to 8 years
- 40 - 60 years
- 20 - 30 years
- 60 - 80 years
- 30 - 40 years
Presence of which of the following genetic abnormalities is a bad prognostic factor for acute lymphoblastic leukemia?
- t (9:22) translocation
- t (12:21) translocation
- t (1:19) translocation
- t (8:14) translocation
- High hyperdiploidy (over 50 chromosomes)
Which of the following cells is is seen in myeloid leukemia on a bone marrow aspirate?
- Primitive myeloblasts
- Promyelocytes
- Metamyelocytes
- Plasma cells
- Lymphoblasts
AML seen with which of the following chromosomal translocations is known to be associated with disseminated intravascular coagulation?
- t (15:17) translocation
- t (12:21) translocation
- t (11:14) translocation
- t (14:18) translocation
- t (16:18) translocation
Which of the following is classically associated with all acute leukemias?
- Bone marrow failure
- Swollen lymph nodes
- Hyperviscosity
- Hemarthrosis
- Massive splenomegaly
Which of the following drugs used in the treatment of acute leukemia causes microtubule arrest and thus prevents the further division of cells?
- Vincristine
- Cytosine arabinoside
- Asparaginase
- Daunorubicin
- Steroids
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
He is my absolute favorite and has helped me TREMENDOUSLY. His lectures are clear and so insightful.
Very clear concise and professional. I like this brand of teaching.