Playlist
Show Playlist
Hide Playlist
Titratable Acids
-
Slides 08 MetabolicAcidosisAlkalosis AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
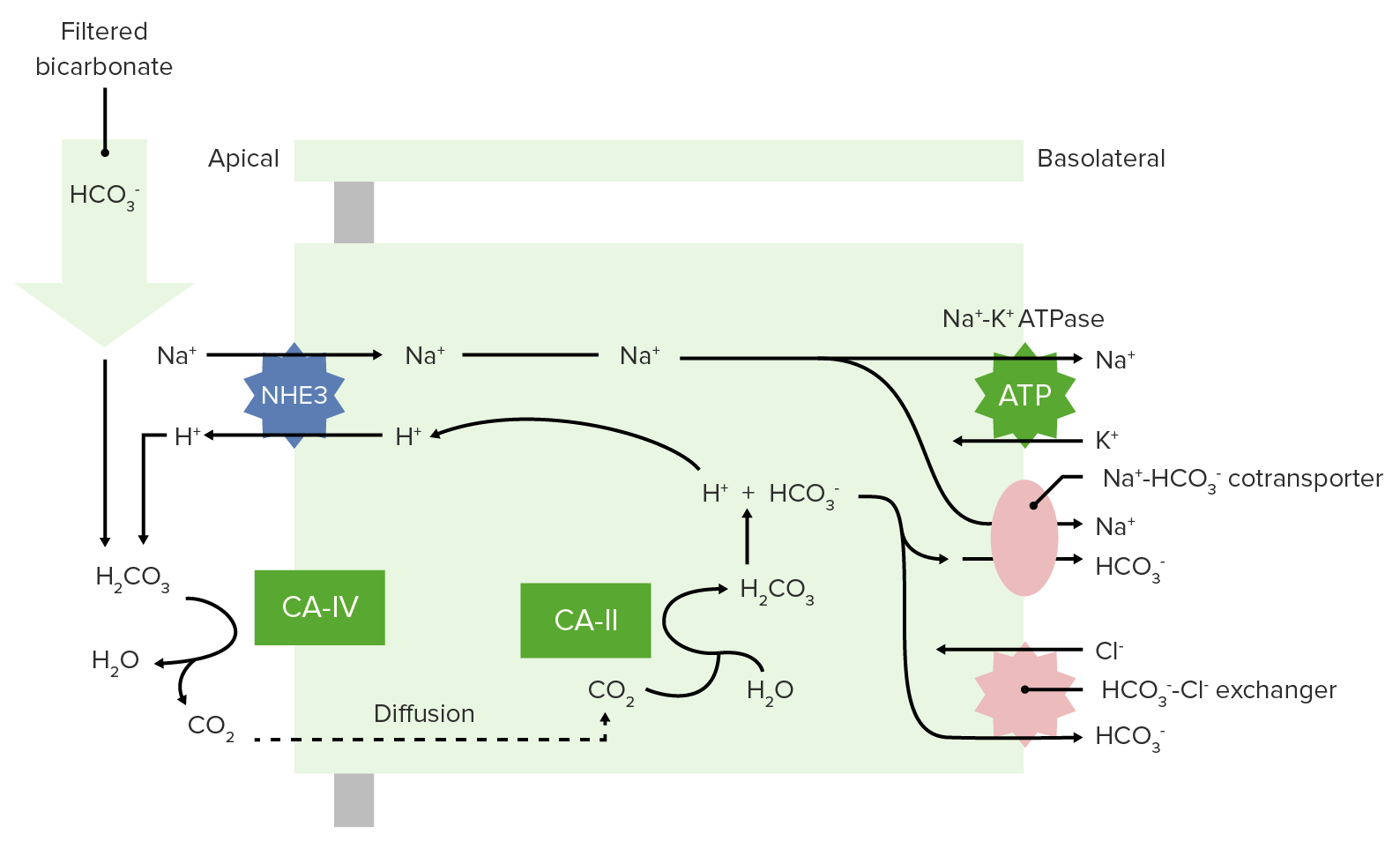
00:01 Now you also have a few other mechanisms to get hydrogen ions out of this apical membrane in the proximal tubule. 00:12 So, we talked about the sodium hydrogen ion exchanger and that was very important for eventually binding into bicarbonate. 00:21 You also have a secondary hydrogen ion transporter, which is a hydrogen ion pump or ATPase. 00:29 So, this HATPase will also push hydrogen ions across the apical membrane. 00:36 No, why is this going to be an important process? Well, remember that a certain portion of the acid that you took in by a non-volatile acids, you are going to have to excrete the hydrogen ion and make a new bicarb. 00:53 So, with the Titratable acids is one of the ways that occurs. 00:57 So, let’s take phosphate as our example. 01:00 So, phosphate will be coming down the renal tubule, and you noticed phosphate has a negative charge on it. 01:08 So, this is HPO4, 2-. 01:14 You will kick out hydrogen ion from the hydrogen ion exchanger or form the V type hydrogen ion pump. 01:22 It will bind then to phosphate. Once it’s bound as phosphate, you can urinate it out. 01:32 So, this is how you can remove a hydrogen ion, is you push it across the apical membrane, have it bind to something and then, have it be removed from the system. 01:45 You don’t have it bind to bicarb, why? Because you want to reabsorb bicarb. 01:50 But phosphate, you can get rid of some of your phosphate. 01:53 But you have to reabsorb your bicarb so you don’t urinate out your bicarb, you bind it rather to one of these Titratable acids. 02:03 The hydrogen ions that then will be left will be in a bound form. 02:10 You may ask, how low can you get your pH of your urine? They say about 4,4 is about the lowest pH of your urine. 02:20 but that could be anywhere from 4,4 up to whatever is in the blood, maybe 7,4 or so forth. 02:27 So, that’s kind of your range of pH of your urine. 02:32 You noticed that phosphate has a pK of around 6,8. 02:36 This becomes important because it’s kind a maximally bind hydrogen ions at it’s pK which is 6,8. 02:45 So, you can utilize this when your pH is very close to 7. 02:51 Some of the other Titratable acids such as urate and creatinine, these have a lower pK. 02:58 Therefore, the pH of the urine has to be lower Before you’re going to be able to utilize these two great extent. 03:06 You also have lactate and pyruvate but those pH’s are actually, or pK’s are below the normal pH That you can obtain in the urine. 03:15 So, these are less important. So, they have very minor roles because of the lower pK values. 03:21 In or with Titratable acids, you can, or make new bicarb in the proximal tubule, the distal convoluted tubule and the collecting duct. 03:33 You make about 40 percent of your new bicarb using titratable acids, phosphate, urate, and creatinine. 03:43 Which one are you going to use? Depends upon the pH of the urine and it’s pK value. 03:50 So, if we look at something like a pH range here for something like phosphate has a wide pH range, so you can utilize that throughout many different pH’s of the urine. 04:02 Something like creatinine, is a little bit tighter. And so, you can’t always use creatinine to bind those hydrogen ions to be able to urinate them out.
About the Lecture
The lecture Titratable Acids by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
Which of the following has a pK of 5.8?
- Urate
- Phosphate
- Creatinine
- Pyruvate
Which of the following is NOT bound to H+ ions thus not routinely eliminated in the urine?
- HCO3
- Phosphate
- Urate
- Creatinine
- Lactate
Which titratable acid has the highest pKa that allows binding of the H+ ions at variable pH?
- Phosphate
- Urate
- Creatinine
- Lactate
- Pyruvate
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |