Playlist
Show Playlist
Hide Playlist
Structure – Amino Acids
-
02 Basic AminoAcids.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
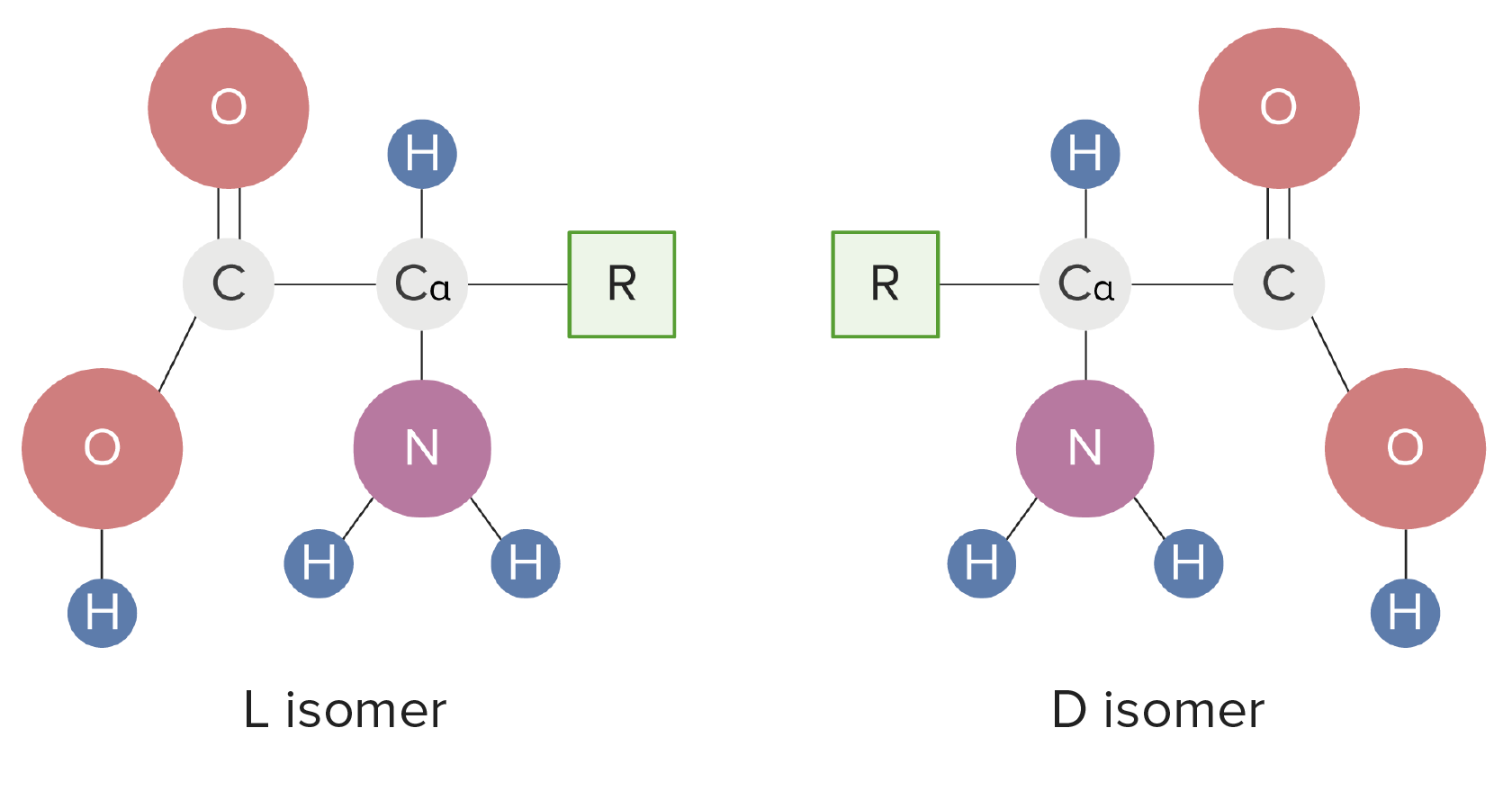
00:01 Proteins are part and parcel of what makes life possible. The diversity of the building blocks of proteins compared to other macromolecules give proteins a rich and diverse array of functions. In this lecture I will talk about proteins starting with the building blocks, the amino acids that make them up and divide them into various groups, essential or nonessential depending upon whether or not they can be made by the organism. I'll discuss the basic structure and stereochemistry of each amino acid and how the side chains of each amino acid give it the individual characteristics that it have. Last I'll give the properties and talk about the ionization of the amino acids found in proteins. 00:41 Proteins, we can describe as the workhorses of the cell. They perform all the essential functions that cells need to stay alive. These include catalysis, catalyzing of the reactions that happen, signaling the process whereby cells and part of an organism can communicate with cells in another part of the organism. The structure of proteins such as the fibrous proteins found in our hair and our nails arises from interesting features within individual protein molecules. Last, proteins are very important for the generation, creation and storage of energy. All proteins on earth are comprised of about 20 amino acids. The 20 amino acids are most commonly found in every organism on earth. A 21st amino acid known as Selenocysteine, in some cases found in a few rare proteins. 01:36 Amino acids can be divided into various categories, one of the categorization schemes is divide amino acids into essential and nonessential groups, depending upon whether or not the organism can synthesize the amino acid within its cells. Essential amino acids are those amino acids that the cell needs to have in its diet because it can't make those itself. Nonessential amino acids are amino acids that cells can synthesize. Now, the categorization of essential versus nonessential varies from one organism to another and it even varies a little bit with the age of the organism, for example, humans have different essential amino acids as adults than they do as children. 02:23 Amino acids that are found in proteins we call alpha amino acids, and they get this name because every amino acid that's used in proteins has a special carbon call the alpha carbon seen here in green in the center of the schematic diagram. All 20 of the amino acids can be drawn in the same schematic scheme. Now there is nomenclature that I want to go through here that will help you to better understand the amino acids in the proteins. 02:51 Every alpha carbon is attached to an alpha carboxyl group, as shown here. And every alpha carbon is also attached to an alpha amine, these two giving the alpha amino acids their name. The alpha carbon is in addition attached on the top to a hydrogen as you can see here and on the left to an R group. The R group is what gives an amino acid its characteristic, functions, shape, structure and properties. 03:20 Now, to show you an actual amino acid according to the scheme that we're using here, I want to show you cysteine. So if we look at the organizational scheme on the right that was on the last slide and compare to cysteine, we can see for example the various things. 03:34 For example, cysteine has an alpha carbon as seen here, an alpha carboxyl group, an alpha amine and an R group that in this case contains a sulfhydryl. Not shown on this figure, but present on the alpha carbon is a hydrogen above the alpha carbon. 03:55 Here is phenylalanine. Phenylalanine for example, has an alpha carbon, an alpha carboxyl an alpha amine and it has a side chain and in this case contains a benzene ring. Again, the alpha carbon contains a hydrogen above it. 04:13 Because the alpha carbon is attached to four different groups it gives the alpha carbon some properties that are important to understand. You learned in organic chemistry for example that if a carbon has four different molecules attached to it, that there are two ways in three-dimensional space that those atoms can be organized around the alpha carbon. 04:36 This shows for example the sugar D-glyceraldehyde and L-glyceraldehyde, two different forms of a sugar that contain a carbon that have four different groups attached them. 04:46 This is known as an asymmetric carbon and it’s shown in the green circles seen here. 04:52 Now there are four different things attached to this and because of this, there's two ways that they can be organized. I've drawn the blue and the yellow arrows to show how it is that two groups, for example, are organized here. We can see under the blue arrow on the left that the gray ball is projecting towards the viewer whereas the orangish ball in the back is projecting away. In the L isomer, these two positions are reversed, we see the orange ball coming to the front and the gray ball moving to the back. 05:21 Amino acids also are designated by the D and L designation that are used for sugars. Interestingly, almost all of the amino acids made by living cells are in the same configuration, that of the L configuration. Now, this is interesting because if you take for example, a test tube and you make amino acids chemically in a test tube that are not being produced by the enzymes of a cell, you get a mixture of 50% D and 50% L. The reason that we get only L exclusively in living cells is because living cells use enzymes to make amino acids, and those enzymes have a three-dimensional specific structure that will only allow the synthesis of one of the two different forms being present. Now this turns out to be really interesting and useful because we can then tell if we analyze an amino acid whether it has a mixture of D and L, or only L, or only D for that matter, because a bias one way or the other would suggest it was made by an enzyme and therefore made by a living cell. This is used for example when meteorites fall to earth and they contain amino acids and scientists are very interested in understanding, were those amino acids produced biologically, or produced by natural chemistry.
About the Lecture
The lecture Structure – Amino Acids by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following molecules are considered primary building blocks of proteins?
- Amino acids
- Nucleotides
- Carbohydrates
- Fatty acids
- Nucleosides
What are essential amino acids?
- They are amino acids that we cannot make but must be obtained from one's diet.
- They are amino acids that we must make from other amino acids.
- They are amino acids that we cannot make but must obtain from the bacterial flora of our GI tract.
- They are amino acids that are essentially in abundance in the environment
- They are amino acids that are made readily in the body.
Which of the following statements about alpha-amino acids is NOT true?
- The alpha carbon has a double bond with an oxygen atom.
- Every alpha carbon is attached to an alpha carboxyl group.
- They all have an R group.
- They are the ones found in proteins.
- Every alpha carbon is attached to an alpha amine.
Customer reviews
4,4 of 5 stars
| 5 Stars |
|
9 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
1 |
I loved this lecture! Everything was so well explained: simple, straightforward, and I especially love few external tidbits thrown in such as the meteorite portion. It helps to remember these facts. Thank you!
this video is short and easy to understand. Thanks Teacher
No explanation at all just reading , sorry for that , No interaction
two stars just for the effort. But its ore like reading fro book. Nothing different. Very boring. No interaction.