Playlist
Show Playlist
Hide Playlist
Rct: Advantages and Disadvantages – Interventional Studies (Study Designs)
-
Slides 08 InterventionalStudies Epidemiology.pdf
-
Reference List Epidemiology and Biostatistics.pdf
-
Download Lecture Overview
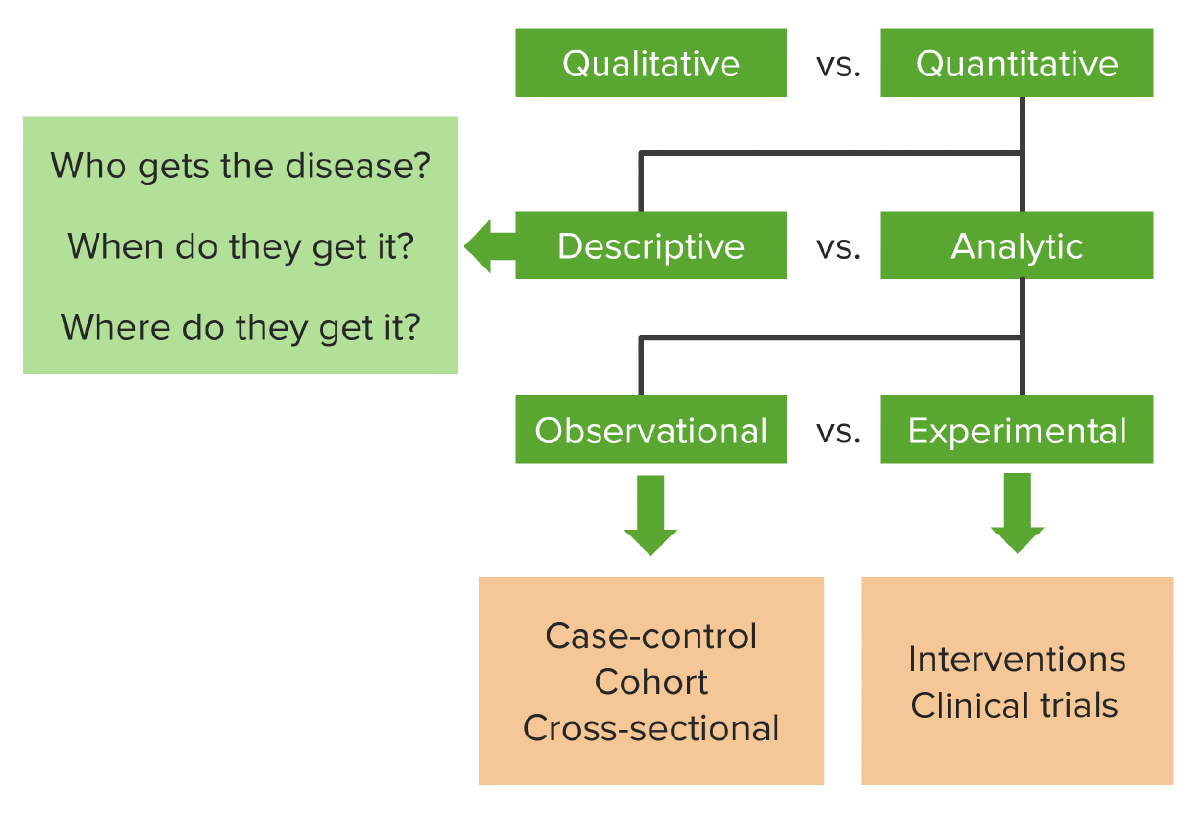
00:01 So let's go through some of the advantages and disadvantages of RCTs, because I mentioned they are gold standard, I mentioned how much we love the RCT and how much we rely upon it, but there are some disadvantages. First of all, the biggest advantage to an RCT is that because it's controlled, the 'C' is controlled, or the 'C' is clinical, depending upon your perspective, I can control the extraneous variables that are in my study. I can make sure that I know that nothing else caused the outcome because I've selected these individuals for their characteristics. On the other hand, it's ridiculously expensive, sometimes millions of dollars to run one of them. Also it doesn't represent the real world, what do I mean? Well if I have selected individuals for certain characteristics and I'm controlling what they're exposed to, sometimes in literally a clinical hospital environment that prevents other people from being involved, that doesn't represent the true post-market environment that the drug or therapy is going to be tested in once it hits the market. So maybe my RCT isn't giving me sufficient real-life knowledge that I can apply. Also the great advantage of doing an RCT is that we can establish a temporal relationship with absolute clarity. I know for a fact that the drug came before the change, I know for a fact that the smoking came before the cancer, if indeed I did an RCT on smoking and cancer, which by the way I can't ethically. 01:26 So the temporal relationship is critical to helping establish causality. Also if I do blinding, and we'll talk about blinding in a second, then it's difficult to argue with the conclusions that I draw within the confines of the study. The internal validity which we will define later on, is pretty secure if the RCT is well-designed and blinded. Ethically however, RCT is problematic. So think about again the possibility of running an RCT on smoking and lung cancer, I'd have to randomly select individuals who are compelled to smoke for many years to see if lung cancer manifests. It's not ethical to do that to people. Also very often if I'm testing a life-saving drug, I'm giving it to people who probably need it, but the control group is not getting that drug. Is it ethical to have people in a study that I know need treatment and I'm not giving them the treatment that I suspect will make them better? That's required for a control group, I have to deny some people the treatment. 02:28 Is that ethical? Let's talk about randomization now, that's the big 'R' in RCT and that's the thing that we're so excited by all the time, because the 'R' gives us a lot of power and a lot of logical confidence. So in a randomized controlled trial, participants are assigned by chance rather than choice, to either treatment group or the control group. Remember, in a cohort study they are choosing to do something, a behavior, an exposure. In an RCT, I'm assigning them as the researcher to one group or another and that assignation, that assignment, is based upon randomization. And again, the classic randomization design is a coin toss, heads you go in this group, tails you go in that group. In reality we don't use coin, we use far more advanced randomization techniques, but the coin toss is a good example for helping you understand how it works. Why do we randomize? We randomize to help reduce the chances of selection bias, you recall selection bias from our lecture on biases. How does this work? Think about this; think about if I know that the drug I am testing works differently on men than on women, I'll make sure that I have equal numbers of men in my control group as in my therapy group. If I know that my drug works differently on old people and young people, then I'll only test it on old people, or I'll only test it on young people, or I'll make sure that I compare my treatment and control groups using only age as a factor. 03:56 In other words, the factors or variables that I know are confounding my relationship, I can control for, but what about the ones that don't know about, that's the beauty of randomization. 04:08 Things that I don't know about may be affecting the relationship between my exposure and my outcome. If I randomly allocate the group that subject gets put into, then I can be well assured that those factors that I cannot control for are also randomly and equally distributed, I hope. When I say I hope I mean I'm relying upon the magic mathematics to give me a very good probability that those characteristics are equally distributed. That's the power of randomization. So randomization, when we do it, each participant that enters my study has an equal chance of being either in this group or in that group. If they have a higher chance of being in another group, then my allocation process is biased. It does not presume that all the participants are the same. Unfortunately randomization doesn't guarantee equivalence, what does that mean? Well simple randomization, like flipping coin, doesn't guarantee I'm going to have equal numbers of people in both groups. Think about this, if I'm flipping a coin, heads goes in this group and tails goes in that group, it's entirely possible I flip heads eight times in a row out of 10 people. Now I've got eight people in this group and 10 people in the other group. That's not great. 05:23 Remember, coin flipping isn't what we always. There are more complicated ways of randomization to account for this, but simple randomization does not guarantee equivalence or balance between the study arms, as we call them. So when we randomize, what we're looking for is to eliminate bias, primarily selection bias. We hope to have balance between the arms with respect to certain variables, like gender and sex and age and all those other things, but especially the ones we didn't even know about. And remember that randomization also forms a basis for a lot of statistical testing, all the tests we might want to apply, assume that my data is somewhat randomly distributed.
About the Lecture
The lecture Rct: Advantages and Disadvantages – Interventional Studies (Study Designs) by Raywat Deonandan, PhD is from the course Types of Studies.
Included Quiz Questions
Which of the following questions would be better studied by an observational type study design rather than a randomized control trial?
- Does IV drug abuse increase the risk for HIV infection?
- Does harm reduction education decrease HIV infections among IV drug users?
- How does a novel medication regimen compare to the established treatment protocols for the management of HIV infection?
- All of the questions would be best answered using an observational type of study design
- All of the questions can be answered ethically using a randomized controlled trial
What is the main disadvantage of randomized controlled trials?
- External validity
- Internal validity
- Takes a long time
- Not enough power
- Ethically challenging
Which type of bias is best avoided by randomization?
- Selection bias
- Confirmation bias
- Question-order bias
- Recall bias
- Misclassification bias
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |