Playlist
Show Playlist
Hide Playlist
Problems With Penicillins – Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
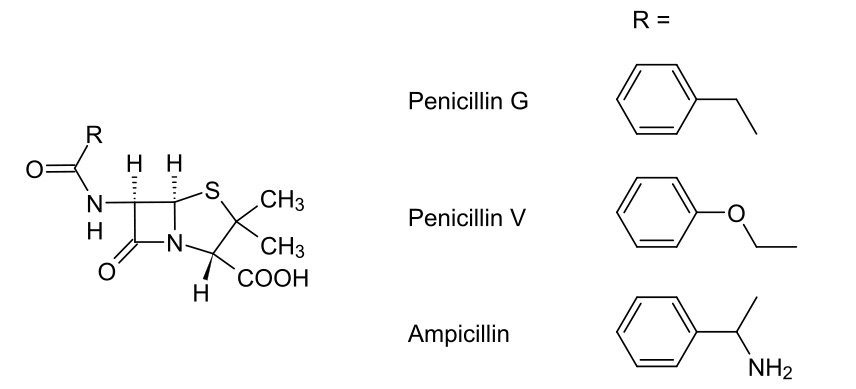
00:00 So, that’s brilliant. But, there are problems. 00:04 The problems stem from the original penicillin that we look at: benzylpenicillin. It was acid sensitive. It was not active over a wide range of bacteria and it was also sensitive to these additional enzymes produced by certain resistant kinds of bacteria called beta-lactamase. 00:22 And what medicinal chemists have set out to do is to try and overcome these disadvantages through making a wide variety of penicillin analogues actually using rational chemistry to try and improve upon the pharmacokinetic properties and pharmacodynamic properties of these. So, let’s have a look at those problems and solutions in a little bit more detail. 00:46 We discussed actually part of this earlier on in the module about the problem of acid sensitivity. So, this is just going to be a brief recap in this case. 00:55 In the presence of catalytic amounts of acid, intramolecular hydrolysis of the beta-lactam group can occur. I will not go through the full method and full mechanism since we have covered that earlier on in this module. But, just to reiterate that where you’ve got this nucleophilic oxygen as part of the sixth-position amide, this can kick in, open up the beta-lactam ring, thus splitting up the beta-lactam four-membered ring system and rendering our penicillin now completely inactive. And this is the problem with oral bio-availability of penicillin G or benzylpenicillin. 01:33 A way around this was, of course, to actually add an electron-withdrawing group in the R position. And this is shown here. This is one of the earlier orally bio-available penicillins, penicillin V, where in this scenario, the R group attached to that carbonyl as part of the amide in the sixth position is electron withdrawing. 01:54 As you can see from this arrow, what this means is it pulls electron density away from the carbonyl-carbon making it less nucleophilic, thus producing its propensity to then attack the carbonyl of the beta-lactam ring. The electron density ultimately is on the carb-... 02:11 is reduced on the carbonyl and therefore, it can be given orally. And it should be stressed that, if you were to see the structure of an unknown penicillin and you should see an electron-withdrawing group directly attached to that sixth position, you should be able to say confidently that it’d most likely be orally bio-available. 02:32 The other problem we have is narrow spectrum of activity. Benzylpenicillin and phenoxymethyl penicillin, penicillin V, are useful mainly against Gram-positive bacteria. And this prompted the development of ampicillin and amoxicillin, the general structure for which is shown here. 02:49 As you can see, we have a couple of slight variations. Instead of having an oxygen in the case of penicillin V neighbouring our carbonyl group on the amide, we now have an NH2 group attached. In the case of amoxicillin, we also have a, in the fourth position of that benzene ring, a hydroxyl substitution. 03:10 And the reason that this seems to work is by virtue of the fact that polar groups in that position, next to the amide on the sixth position of the bicyclic ring system of the penicillin seem to impart broader spectrum of activity. And this was determined experimentally rather than with any particular rationale in mind. 03:34 Amoxicillin has better activity against Gram-negative bacteria than the other penicillins because it’s related to the ability of the penicillin to cross the cell wall. 03:44 If I cast your minds back to where we looked at the general structures of the cell walls between Gram-positive and Gram-negative, this makes some sense. In the case of Gram-positive bacteria, the cell wall is effectively exposed and so, therefore, is the enzyme peptide... 03:59 penicillin-binding protein. If we look on the Gram-negative side of things, the peptidoglycan was hidden between a layer of lipopolysaccharide. And so, in this particular scenario, it was determined experimentally that incorporating a polar group in that sixth position actually improved the ability to penetrate that lipopolysaccharide layer through the porin substructure. It’s thought that penicillins, which have got those polar groups attached, can more easily pass through porins in the outer membrane, thus accessing the peptidoglycan layer underneath.
About the Lecture
The lecture Problems With Penicillins – Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Introduction of an amine group (NH2) on the 6-amidic site of penicillin is likely to have which effect?
- Improved spectrum of activity.
- Reduced aqueous solubility.
- Greater resistance to bacterial β-Lactamases.
- Reduced risk of intramolecular β-Lactam hydrolysis.
- None of the above.
Complete the following statement. The main challenges associated with the use of the original penicillin as an antibiotic include …
- … the acid sensitivity, narrow spectrum antibacterial activity and failure to inhibit beta-lactamase positive bacterial strains.
- … the ability to degrade the bacterial cell wall at very fast rates.
- … the base sensitivity and broad spectrum antibiotic.
- … the resistance to degradation via beta-lactamase enzymes.
- … the inability to stimulate the binding of penicillin-binding protein with peptide bridges.
Complete the following statement. The presence of catalytic amounts of acids stimulates …
- … the intramolecular hydrolysis of the beta-lactam ring.
- … the polymerization of penicillin molecules.
- … the intermolecular hydrolysis of beta-lactam ring.
- … the intramolecular hydrolysis of thiazolidine ring.
- … the intermolecular hydrolysis of thiazolidine ring.
Complete the following statement. The orally bioavailable penicillins like penicillin V, has an electron-withdrawing group attached to carbonyl …
- … at the 6ᵗʰ position which imparts -I effect and makes this carbonyl group less nucleophilic.
- … at the 6ᵗʰ position which imparts +I effect and makes this carbonyl group more nucleophilic.
- … at the 6ᵗʰ position which imparts -I effect and makes this carbonyl group more nucleophilic.
- … at the 6ᵗʰ position which imparts +I effect and makes this carbonyl group less nucleophilic.
- … at the 5ᵗʰ position which imparts +I effect and makes this carbonyl group more nucleophilic.
Which of the following is NOT a broad-spectrum penicillin?
- Benzylpenicillin
- Amoxicillin
- Ampicillin
- Pivampicillin
- Hetacillin
Complete the following statement. The addition of a polar group at the 6ᵗʰ position of the original penicillin molecule …
- … enables the penicillin molecules to penetrate the lipopolysaccharide layer of gram-negative bacteria through porins.
- … enables the penicillin molecules to penetrate the cell membrane of gram-negative bacteria through ion channels.
- … enables the penicillin molecules to penetrate the lipopolysaccharide layer of gram-positive bacteria through porins.
- … enables the penicillin molecules to penetrate the cell membrane of gram-positive bacteria through ion channels.
- … enables the penicillin molecules to penetrate the cell membrane of gram-positive bacteria through voltage-gated sodium channels.
Complete the following statement. The isolation of pure samples faces many obstacles during the initial phases of drug development ...
- ... due to their susceptibility to hydrolysis.
- ... due to their unstable structures.
- ... due to their inert chemical behaviors.
- ... due to their interlinking with metals.
- ... due to their hydrogen bonding with water molecules.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |