Playlist
Show Playlist
Hide Playlist
Problem of Beta Lactamases – Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
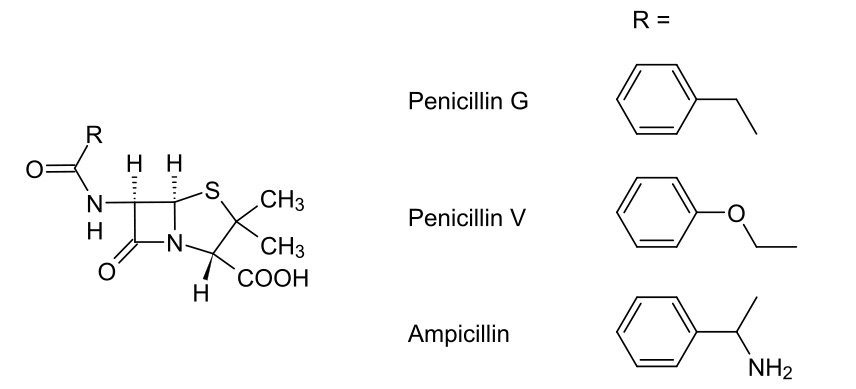
00:01 Now, that’s okay, if you see a polar group attached. So, for example, an amine group or maybe a carboxylic acid group, you should be able to confidently say that it should have a better spectrum of activity than penicillin G. 00:12 Now, I’d like to bring us onto the problem of beta-lactamases. 00:17 Penicillin-resistant bacteria can produce beta-lactamases which catalyse the hydrolysis of that beta-lactam bond, thus inactivating the penicillin. Let us have a look at this example again. 00:30 This is the general structure of a penicillin and beta-lactamases act on it to break open that beta-lactam bond, thus giving us the amine and the free carboxylic acid. The action of a beta-lactamase destroys the antibiotic activity of the penicillin. And what we’ve seen more recently is the... the expression perhaps of more beta-lactamases by bacteria as resistance is gained. This is a real problem in terms of the efficacy of the antibiotics that we would want to use. 01:04 So, what are the ways around it? Let’s move on and discuss this further. 01:10 So, the problem of beta-lactamase-producing bacteria: how can this be overcome? So, to start off with it was important to recognise that beta-lactamases themselves are not very tolerant to steric hindrance. That is to say bulky groups near the side chain amide bond in the sixth position. And this was discovered with the synthesis of methicillin shown here in the bottom left hand side. The presence of those o-methyl groups in the ortho position adjacent to that amide bond in the sixth position of the penicillin actually confer on it a resistance to beta-lactamases. And so, methicillin resistance was considered to be a benchmark of bacteria which actually produced a different isoform of the penicillin-binding protein through the [Unaware 00:24:38]. 02:01 What is interesting to note, however, is that, if you move those bulky groups, one CH2 group, away from the amide bond in the sixth position, you actually create something which goes back to being beta-lactamase sensitive. This is, of course, problematic because it means that that resistance that’s imparted through the bulky groups only takes place if they’re very, very close to that amide. 02:24 Another thing to bear in mind, of course, is that lacking a polar group or an electron-withdrawing group, is also means that not only is methicillin a narrow-spectrum antibiotic for Gram-positive bacteria, but it’s also not orally bio-available. 02:39 So, as I said, methicillin itself is not orally active due to the absence of electron-withdrawing groups in the side chain. And it was this next class of penicillin-based antibiotics which were designed to combat that. 02:56 As you can see from the structure, they possess this ring system known, in this case, as an isoxazolyl ring system characterised by the presence of the nitrogen and the oxygen next to each other. This conveys on it a degree of bulk or steric hindrance which actually serves to inhibit beta-lactamases, but also provides or allows it to engage with the penicillin-binding protein. 03:22 At the same time, the isoxazolyl ring itself serves as an electron sink pulling electron density away from that amide in the sixth position, therefore conveying on it oral bio-availability. 03:35 And so, indeed, you may have actually had some of these to treat an infection. There are flucloxacillin, where you have chlorine and fluorine substituted in the benzene ring and oxacillin, where you have no substitution at all. 03:50 As we said, in the absence of having polar groups such as our NH2 or, for example, carboxylic acid groups, they also have a very narrow spectrum of activity and are not particularly active at all against Gram-negative bacteria.
About the Lecture
The lecture Problem of Beta Lactamases – Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
How does beta-lactamase enzyme inactivate penicillin?
- By catalyzing the hydrolysis of beta-lactam bond to give amine and free carboxylic acid.
- By catalyzing the hydrolysis of thiazolidine ring.
- By catalyzing the polymerization of two penicillin molecules through their beta-lactam and thiazolidine rings.
- By catalyzing the polymerization of two penicillin molecules through their beta-lactam rings.
- By catalyzing the polymerization of two penicillin molecules through their thiazolidine rings.
To overcome the problem caused by beta-lactamases, what kind of changes are introduced in the structure of original penicillin?
- The addition of bulkier groups near the side chain amide bond in the sixth position.
- The addition of -OH group near the side chain amide bond in the sixth position.
- The addition of -Cl near the side chain amide bond in the sixth position.
- The removal of bulkier groups near the side chain amide bond in the sixth position.
- The removal of thiazolidine ring from the structure of the original penicillin.
Why is methicillin a narrow spectrum non-oral antibiotic?
- Because it lacks a polar or electron withdrawing group in its structure.
- Because the methicillin contains a polar group in its structure.
- Because of the presence of an electron withdrawing group in its structure.
- Because of the existence of a metal ion in its structure.
- Because it has a helium atom in its structure.
What is the role of the isoxazolyl group in an antibiotic?
- It provides steric hindrance to beta-lactamase, helps in PBP binding to penicillin and acts as an electron sink.
- It helps the beta-lactamase enzyme to attack thiazolidine ring of penicillin.
- It acts as an electron donor to facilitate autohydrolysis of penicillin molecule.
- It prevents the binding of penicillin binding protein to the peptide bridge.
- It facilitates the binding of beta-lactamase enzyme onto the penicillin binding protein.
Complete the following statement. To improve the spectrum of activity and resistance to beta-lactamases …
- … emphasis is given on structural changes.
- … emphasis is given to remove the lone pair of electrons from the N atom present in cyclic amide.
- … emphasis is given to add an additional lone pair of electrons on the N atom of cyclic amide.
- … emphasis is given to add a lone pair of electrons to the carbonyl group.
- … emphasis is given to remove oxygen from the carbonyl group.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |