Playlist
Show Playlist
Hide Playlist
ph Adjustment: Acid Handling
-
Slides 08 MetabolicAcidosisAlkalosis AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
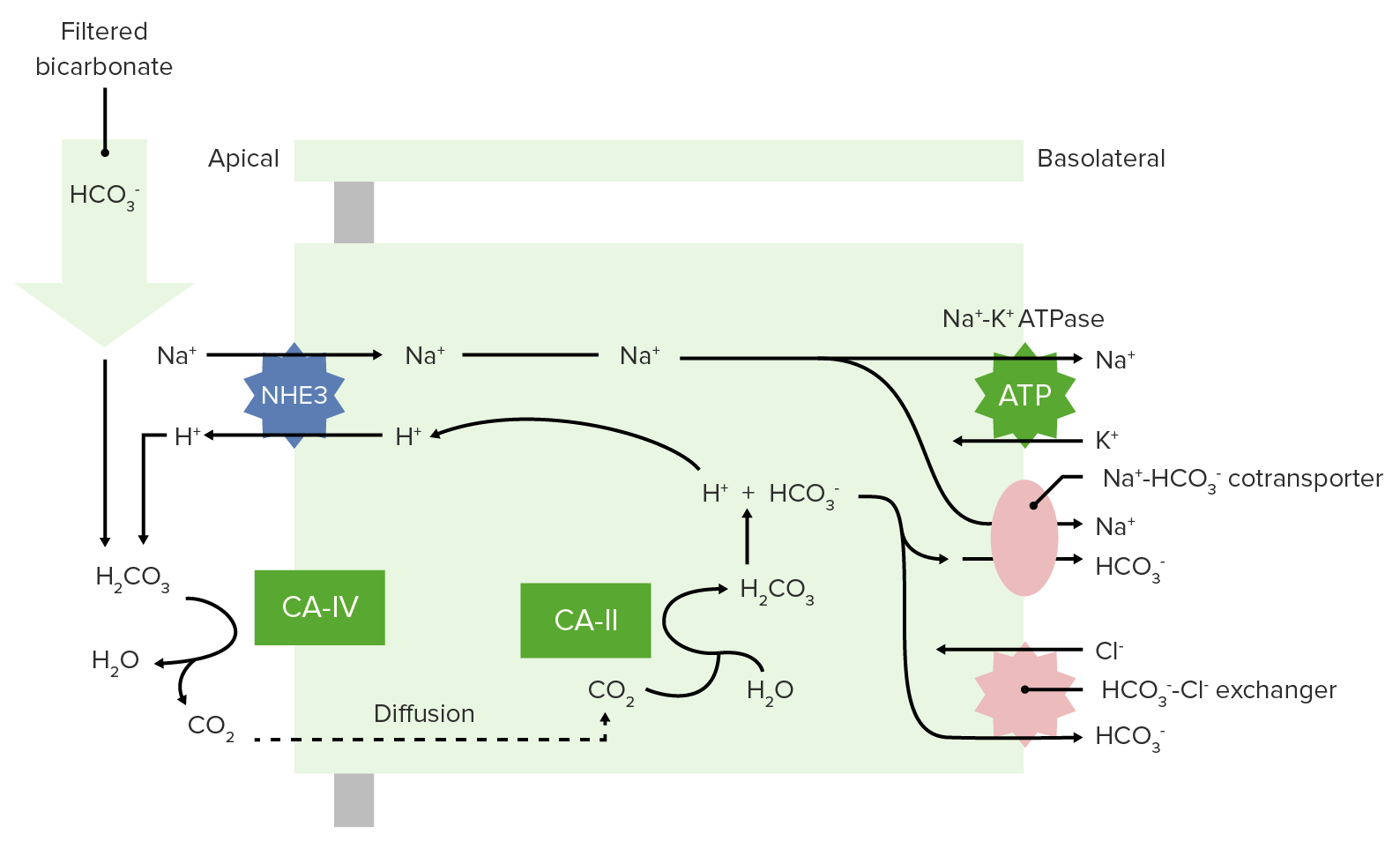
00:00 Now let’s deal with acidosis and alkalosis and how we deal with pH in the body. 00:10 The plasma pH or I’m going to call the extracellular fluid pH is 7,4. 00:17 But how do you get this 7,4 value? Cos again, it’s just an aggregate of all the acids produced throughout the body. 00:26 And then, all the acids that you’ve got to rid of and all the acids you buffered. 00:30 So, let’s go through one by one, where this come from and then deal with what happens to them. 00:37 The biggest thing is metabolism. 00:41 During metabolism you create acids. 00:45 Most of the acids you produce are Volatile or from CO2. 00:51 So, in this case, about 15,000 mM of acid is produced per day by carbon dioxide. 01:00 This is your Volatile acid. This is a huge amount of acid through aerobic metabolism. 01:06 What do I mean by aerobic metabolism? Remember, when you go through glycolysis into the pyruvate dehydrogenase complex through the Kreb’s cycle on an electron transport chain, there were CO2s that were kicked off along the way as you produced the ATP. 01:23 Interestingly, there is also a little bit of acid that’s produced form non-volatile acids. 01:30 Only about 40 mM. So, we think about this 15,000 Volatile, 40 mM were in non-volatile. 01:41 What do you do with these Volatile acids? It’s very easy, you just take CO2 to the lungs and you breathe it out. 01:51 And that 15,000 mM gone. 01:55 You don’t have to deal with it anymore. 01:57 Simply, by breathing out you’ve gotten rid almost all of your acid. 02:04 You can’t though breathe off that 40 mM. 02:08 With these non- volatile acids will not be able to produce CO2, go to the lungs, get breathe off. 02:15 We have to do something else with them. 02:18 Okay, let’s talk through how else we can get acid. 02:22 You have a little bit of exchange of acids and bases in your GI tract. 02:28 So, maybe about 30 mM per day of acids comes from the GUT. 02:35 You combine that with the 40 mM that’s produced during or anaerobic metabolism. 02:42 And that gives you a sum total of around 70 mM. 02:49 You have to deal with that 70 mM. 02:52 The only organ system you have to deal with the 70 mM is the kidneys. 02:59 You have to work through what to do with that. 03:04 They kidneys do two things for you. 03:07 One is it reabsorbs bicarb. 03:10 Remember, bicarb is our primary buffer in the extracellular fluid. 03:15 So, you could see a filtered load of bicarb, might be somewhere around 4220 amount of bicarb filtered per day. 03:28 You reabsorb all of it during the course of the day so you don’t lose bicarb. 03:35 You don’t want to lose your buffer, keep the buffers around. But now you still have this 70 mM. 03:42 You have to do something with that. 03:44 It just so happens that as you buffer the 70 mM of hydrogen ions, you produce 70 mM of new bicarb. 03:55 So, every time you get rid of a hydrogen ion, and you urinated out, you make a new bicarb. 04:02 So, this is where we’re going to deal with today, is how the kidneys deal with those 70 mM of non- volatile acids. 04:11 But let’s also revisit how this process works. 04:17 As you put all the pieces together, know that the GI system is involved but it gives you only a minor amount of the non- volatile acids. 04:28 Metabolism gives you the most non-volatile acids. 04:31 And then, the 15,000 mM of Volatile acid, the lungs just take care of. 04:38 So, we don’t have to deal with that in a great extent because it’s so much as being taking care of from the respiratory system. 04:45 You urinate out 70 mM of hydrogen ion, which is the sum total from metabolism in the GUT. 04:55 When thinking about non- volatile acids, let’s revisit how the body typically deals with acid. 05:02 It handles these non- volatile acids at the level of the kidney. 05:07 So, we need to talk through the kidneys specific mechanisms by which it deals with this non- volatile acids. 05:14 So, the kidneys doing two things for us; reabsorbing bicarb, and making new bicarb while it’s kicking out an acid, non-volatile acid.
About the Lecture
The lecture ph Adjustment: Acid Handling by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
which of the following normally removes volatile acids from the body?
- Lung
- Stomach
- Pancreas
- Kidney
Which of the following organ plays a major role in a removal of non-volatile acids?
- Kidneys
- Lungs
- GI tract
- Urinary bladder
- Pancreas
What is the function of kidney in handling non-volatile acids?
- Reabsorbing bicarbonate and making new bicarbonate as it replaces the non-volatile acids.
- Reabsorbing H+ ions and making new H+ ions as it replaces the non-volatile acids.
- Reabsorbing H+ ions and making new bicarbonate as it replaces the non-volatile acids.
- Reabsorbing bi-carbonate and making new H+ ions as it replaces the non-volatile acids.
- Reabsorbing bi-carbonate and making new bicarbonate in addition to the non-volatile acids.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
The mechanism just summarises all the information into one picture