Playlist
Show Playlist
Hide Playlist
Membrane Potential: Ion Concentration
-
Slides 04 MembraneExcitability GeneralPhysiology.pdf
-
Download Lecture Overview
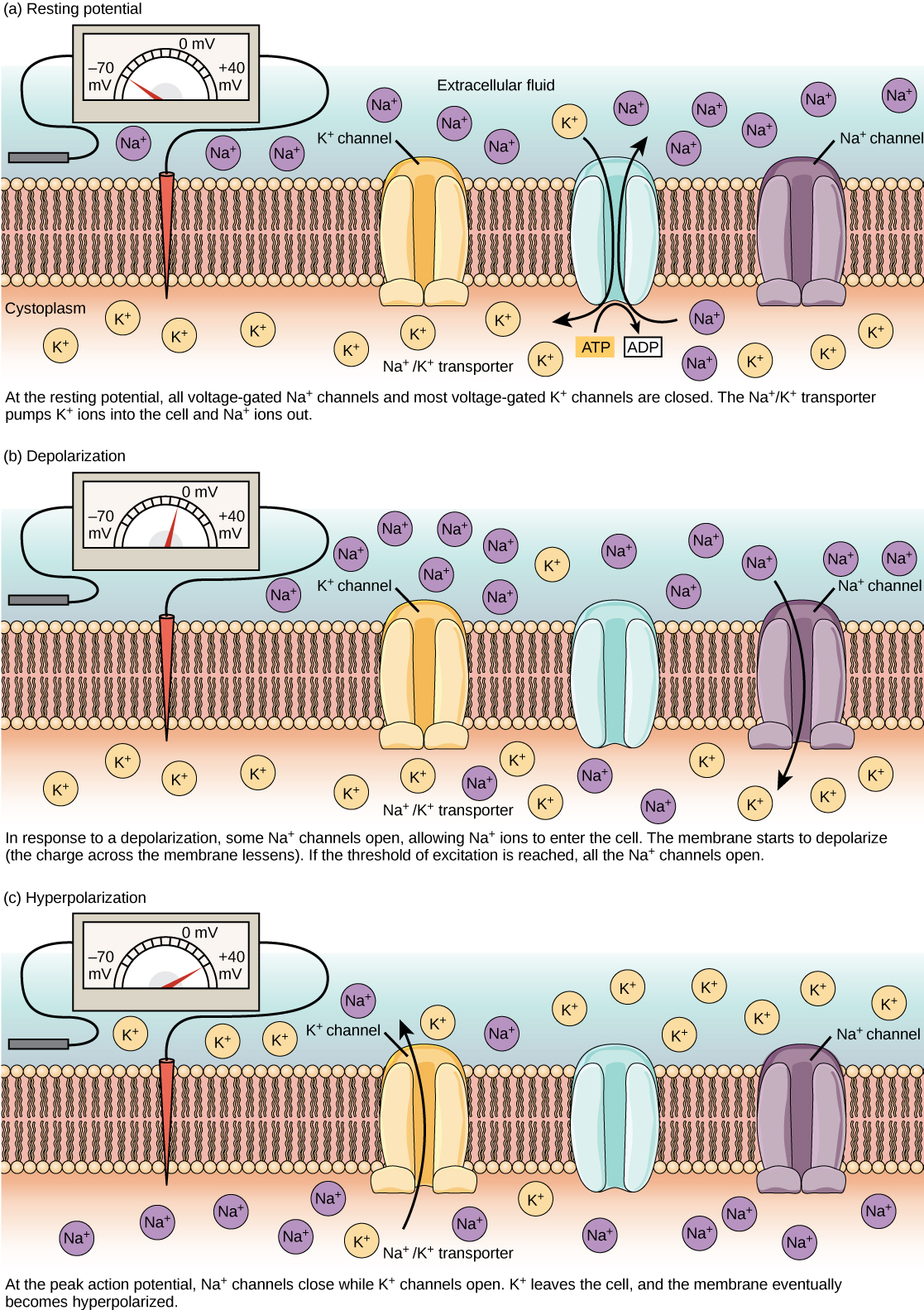
00:01 Membrane excitability. 00:05 To understand how excited a membrane can be and I know you’re excited too to learn about membrane excitability. 00:14 What we really need to do to look at the current changes across any particular membrane is you need to use a recording electrode. 00:24 Simply, you put a recording electrode one side of the membrane and then on the other, or you put it inside the cell and then ground it. 00:33 The potential across the membrane allows for some signals to potentially be sent. 00:40 And interestingly, the signal between or the difference between the membrane and the outside the cell oftentimes has a voltage potential. 00:50 This voltage potential is determined by the ion concentrations inside and out of the cell. 00:58 So by knowing the ion concentrations, you can get inside into this voltage change. 01:07 How does this work? Well, you need to know a few things. 01:12 So I’m going to use a couple of theoretical examples first and then we’re going to go into some practicals. 01:18 Theoretically, if we had this bath and we had a divider in the middle of the bath that didn’t allow any ions to travel through. 01:28 Let’s just label one of them ECF, which is representative of the extracellular fluid, and the ICF as representative of the intracellular fluid. 01:39 You put an electrode on one side, electrode on the other side of the bath. 01:46 In this case, we’re using potassium and chloride. 01:50 In our example here, the number of positive and negative charges is equal. 01:58 Now, I know if you’re looking at these too bad, you’re telling me, how is that equal? What I’m saying here is the number positives equal the number of negatives on both sides of the bath not that the one side of the bath has a lot more molecules on than the other. 02:16 It’s just the sum of the charges that is important, not that particular ion number. 02:24 So when there’s no charge imbalance across this particular membrane, there’s no voltage. 02:32 So even though there’s a different number of ions in one side than the other, there’s no voltage. 02:39 Let’s now contrast that in another condition in which the membrane between the baths now have little channels in them. 02:48 And these channels are going to allow some of the ions to travel through and let’s say those channels were selective enough to only let the positive charged ions through. 03:02 Yeah, it’s exactly what you would do, you’re only going to let positive people through the door into your bath, you’re going to keep the negative ones over on the other side. 03:11 If you have the positive charges, go to one side. 03:18 If you’re recording in the opposite side, you’ll have more negative charges there and so membrane potential will decrease if you’re looking at it from the ICF side. 03:30 If you are looking at it from the ECF side, membrane potential would have increased. 03:36 So it matters where your recording electrode and where your reference electrode is located. 03:43 If you’re recording electrodes in one side, you’ll only be able to determine the changes in that side in comparison to the other.
About the Lecture
The lecture Membrane Potential: Ion Concentration by Thad Wilson, PhD is from the course Membrane Physiology.
Included Quiz Questions
A recording electrode is in bath A, a reference electrode is in bath B, and an ion-impermeable membrane separates the baths. An ion channel opens in the membrane to allow only positive ions to flow into bath A. What would be the most likely result?
- The voltage on the B-side would become more negative.
- The voltage on the A-side would become more negative.
- The voltage on the B side would become more positive.
- Voltage would stay the same since ions are too small to make a difference in charges.
- Voltage changes can only be produced by two ions at a time.
What determines a membrane potential?
- The difference in ion concentration between the inside and outside of the cell
- The presence of recording electrodes
- The presence of a membrane between two solutes
- The presence of Intracellular fluid and extracellular fluid
- The presence of selective pores in the cell membrane
Which of the following determines total membrane potential?
- The sum of the number of charges across the membrane.
- The number of ions on each side of the membrane.
- The number of anions on each side of the membrane.
- The number of cations on each side of the membrane.
- The presence of a membrane separating two solutes.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
nice and excellent lecture ..................found it easy to understand...............................thanks to Dr Thad Wilson for sharing his expertise and knowledge..............................also thank u lecturio