Playlist
Show Playlist
Hide Playlist
Lipophilicity – Pharmacokinetics and Pharmacodynamics
-
Slides 12 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
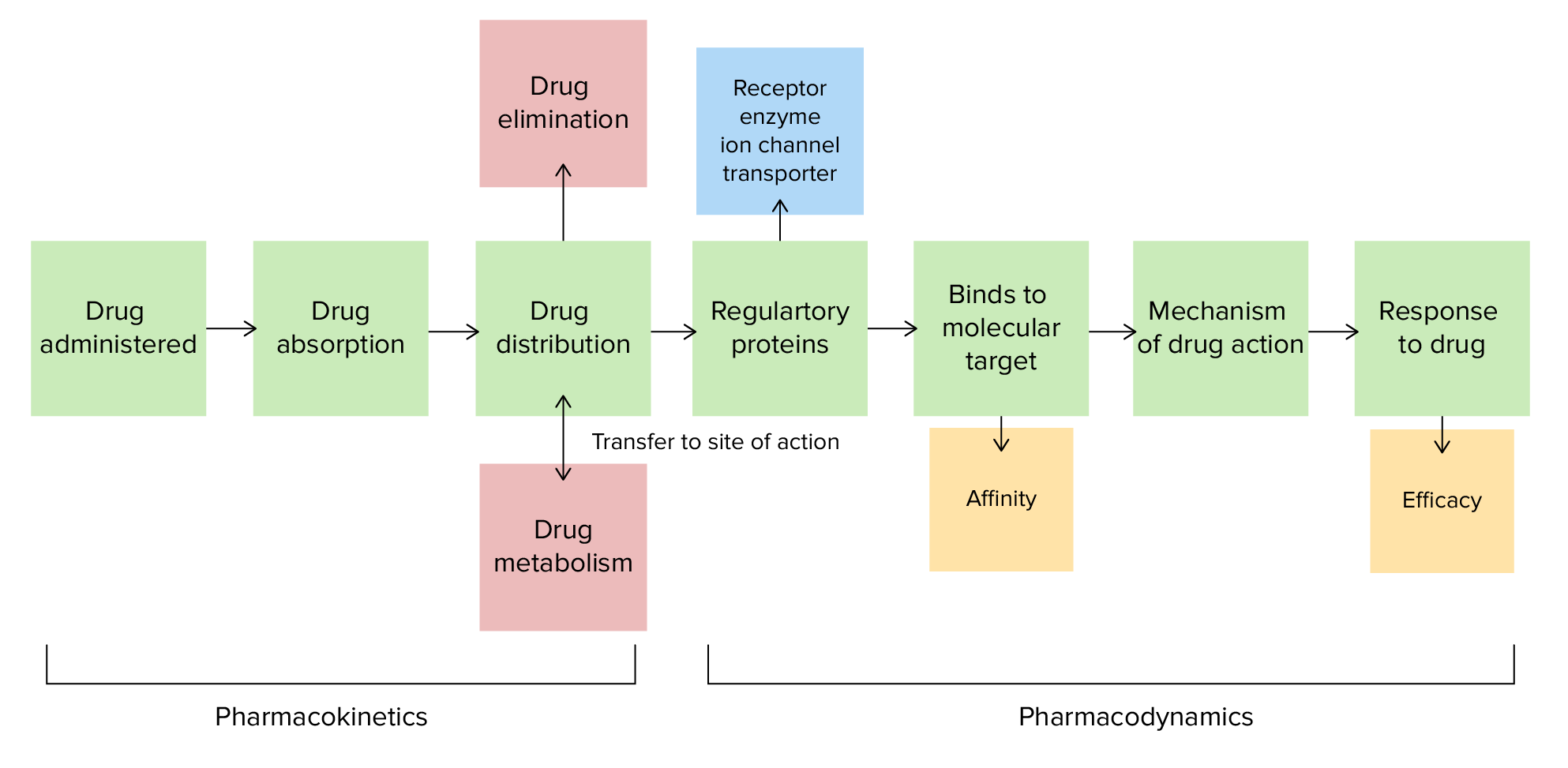
00:01 In general, the higher the lipophilicity of a drug, the higher its membrane permeability and therefore, the higher its metabolic clearance, which is through the first-pass effect. Therefore, it is important to balance lipophilicity with metabolic susceptibility. So, modification of the drug structure can alter the lipophilicity of the compound. Molecules can be made more lipophilic by masking a polar group such as an amine or an alcohol or a carboxylic acid with an alkyl or acyl group. 00:35 This, however, may lead to reduction in activity, if that polar group is actually required for interaction with that receptor. In this scenario, a temporary masking group, prodrug, which we’ll discuss a little bit later on, is something that has to be considered. 00:54 An example of such a prodrug approach would be the conversion of a phenol or an alcohol into an ester. The ester can then be hydrolysed by esterases within the body, thus releasing the active component from the prodrug. Let’s have a quick look here at these two examples. Tetracycline is an antibiotic. It’s an antibiotic that interferes with protein biosynthesis within prokaryotes or bacterial cells. It is not particularly well absorbed. 01:25 However, by substituting a chlorine group onto one of the… onto the only benzene ring in the structure, you can improve the lipophilicity and therefore, the absorption and oral bioavailability of chloretetracycline. Sometimes, the reverse is true. The metabolism of something which is very lipophilic is actually very… causes there to be less bioavailability. 01:48 It seems there would be less available to actually work in the area where it’s supposed to. And so, in this scenario, polar groups can be added in order to render it less prone to metabolism via the first pass. An example here would be clotrimazole and fluconazole. Note the lipophilicity of the first, Canestan, and the fact that by adding a polar group, in this case an OH group here. In the case of fluconazole, we are actually reducing the lipophilicity, making it more polar. 02:24 Let’s have a quick look at another one, an anti-viral, aprenavir: poor aqueous solubility and therefore, a large number of excipients are required resulting in a dose of around 8 capsules twice daily. This is obviously a considerable burden in terms of patient compliance. However, if we incorporate a phosphate group into the molecule, giving us the prodrug fosamprenavir, this dramatically increases the aqueous solubility, resulting in a higher drug load in the tablet and therefore, greater bioavailability of the anti-viral itself. 03:04 The phosphate group removed by… is removed by phosphatases yielding the parent drug and here, we have the structures. They may appear complex, but if we look to the right and see aprenavir, we can see that there is a secondary alcohol there joining the two aromatic systems. 03:23 If we convert that into a phosphate group, such as in the case of fosamprenavir, we find that those 2 OH groups attached to the phosphorus are actually ionised at physiological pH and exist as O- in both cases. Of course, going back to where we were talking about ion-dipole interactions, this means that it’s possible for the negatively charged O- on the fosamprenavir to interact strongly with water molecules. This increases, of course, the solubility because it is polar. By obviously increasing the solubility, this increases the bioavailability of the drug when taken and reduces the number of tablets that need to be given to a patient. Ionisation. 04:12 Most drugs are either weak acids or weak bases containing, as they do, they are either carboxylic acids or, for example, amines. And so, therefore, they exist in equilibrium as both unionised and ionised forms. Unionised molecules, molecules which are neutral, find it easier to cross through the cell membrane. The cell membrane, if you recall, is effectively a phospholipid bilayer. It is, to all intents and purposes, lipophilic. And to penetrate through the cell membrane, it is often the case that you need neutral molecules for passive diffusion to be successful. Ionisable groups can actually be masked. Going back to what we said before, for example, converting a carboxylic acid into an ester, as in the case of a number of prodrugs that we’ll come on to in the next lecture. However, other factors also affect the amount of active drug being absorbed. 05:11 Acid sensitivity, which we’ll see in a second, can be a major problem for some drugs and will have a dramatic effect on the amount of active drug absorbed.
About the Lecture
The lecture Lipophilicity – Pharmacokinetics and Pharmacodynamics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
How does the lipophilicity of a drug affect its efficacy?
- A higher lipophilicity leads to a higher membrane permeability and metabolic clearance of the drug.
- A higher lipophilicity leads to a lower membrane permeability of the drug.
- A higher lipophilicity leads to a lower metabolic clearance of the drug.
- A lower lipophilicity leads to a higher rate of absorption into the circulatory system.
- A lower lipophilicity leads to a higher rate of desorption into the food.
Which of the following statements is NOT true?
- If given in its unaltered form as an oral medication, tetracycline will effectively interfere with the protein biosynthesis process in eukaryotic cells.
- The conversion of tetracycline into chlortetracycline leads to enhanced absorption and bioavailability.
- The introduction of a polar group reduces the lipophilicity of the drug and renders it less prone to metabolism.
- The phosphate group in the molecular structure of “Fosamprenavir” enhances the aqueous solubility and greater bioavailability during anti-viral therapy.
- Enzymes like esterase or phosphatase play a significant role in the conversion of a prodrug into an active form.
Customer reviews
2,1 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
2 |
| 1 Star |
|
5 |
9 customer reviews without text
9 user review without text