Playlist
Show Playlist
Hide Playlist
Introduction – Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
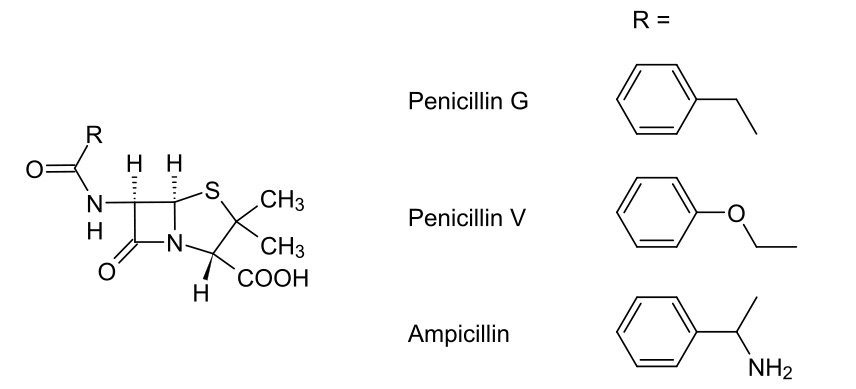
00:02 Now, we come onto to the final lecture in Module 4. And this relates to beta-lactam antibiotics. 00:09 Hopefully, you’ll be able to appreciate, having gone through a number of functional groups, some of the structural changes that we make to these antibiotics in order to improve their spectrum of activity, their resistance to beta-lactamases and also, as we will see, their oral bio-availability. 00:29 As you’ll probably be aware, antibiotics are one of the most-frequently prescribed medications. They achieve this by selective toxicity to the pathogen which is a key concept in antibiotic therapy. And approximately, half of the world’s sales of antibiotics are due to the beta-lactam class of antibiotic, which we’ll be discussing today. 00:54 They all work in pretty much the same way, that is to say, they disrupt bacterial cell wall synthesis. And they are good examples of enzyme inhibitors that are irreversible. 01:09 Bacterial cell walls offer a selective target for therapy in the case of bacteria because normal healthy eukaryotic cells, of course, do not have cell walls, consisting of a cell membrane alone. 01:22 So, let’s touch upon penicillins. 01:26 Before I go into the full structure of penicillins, I’d like you to pay attention to the sub-structure I’ve shown on the right hand side. This, you should recognise from Module III, is an amide. Note, we have a carbonyl-carbon to which is attached a nitrogen. But, it is a particular type of amide. It is cyclic. And cyclic amides are known as lactams. And it is this beta-lactam, because we have an alpha and a beta carbon separating the carbonyl and the nitrogen components, that are the core component of penicillins. 02:05 I’ve shown here, at the bottom right hand side, the general structure for penicillins. 02:12 And these penicillin class were first discovered in 1929 by Fleming, consisting of a beta-lactam ring, which is a cyclic amide, fused with a substituted thiazolidine ring. This is that five-membered ring that effectively contains the nitrogen and sulphur at the same time, thiazolidine ring. 02:34 What’s interesting is that, whilst it was discovered in 1929 by Fleming, it was really the Australian Florey and also the German chemist Chain that actually led to the drugability of these molecules. And the problem lay in the fact that it was difficult to isolate pure samples of these because of their propensity for hydrolysis. And, as we’ll see, that propensity for hydrolysis is what caused some of the issues with oral bio-availability. 03:03 As you can appreciate, where, for example, we have a beta-lactam group, such as that shown at the top, we have a very strange ring structure. We have a very small ring structure and therefore, the desire for this to open overcomes in many respects the general lack of reactivity found in amides. 03:23 From a biosynthetic pathway, we’re looking at the formation of these from cysteine and valine as the amino acids. And so, when these are synthesised by a particular penicillium mould, they are synthesised from cysteine amino acid and valine amino acid. And you can see their structures there highlighted in green and red. 03:50 Now, originally, penicillins were produced by the fermentation of penicillium chrysogenum. 03:59 As you can see, they have the general structure shown in the top right-hand corner. Now, I’m not necessarily going to go through the full nomenclature of penicillins because it’s rather unnecessary. Suffice to say, the majority of changes which impart a change in pharmacokinetic profile take place in the sixth position. 04:19 The sixth position is marked here as the alpha position in the beta-lactam ring. And so, you should pay attention to the changes in that region as we come along, continue this lecture. 04:29 One of the first penicillins to be obtained was so-called penicillin G or benzylpenicillin. 04:36 And this was actually obtained from culture when a phenylacetic acid was added to the medium, thus resulting in the formation of the benzylamide derivative in that sixth position. 04:51 Addition of a phenoxymethylpenicillin actually resulted in penicillin V, which was one of the first orally bio-available penicillins. And this was achieved if phenoxyacetic acid is added to the medium. 05:06 Finally, it wasn’t until 1958 that the first full synthesis of the precursor to all of these 6-aminopenicillanic acid, or 6-APA, was isolated. This, as you can see, is where we have the free primary amine instead of that substitution pattern where R = H, in the case of our structure in the top right hand side. 05:31 As soon as this penicillanic acid was made available, an entire raft of thousands of analogues were made through the partial synthesis, the semi-synthetic penicillins. For example, once you have that free amino acid, it’s possible to create the amide with a wide variety of different acid chlorides and acid anhydrides. 05:55 Note the way the reaction works in this case, it’s the same as the addition-elimination reaction we’ve covered in Module III, which is to say the lone pair on the nitrogen in the sixth position, as you can see here on the left hand side, attacks the carbonyl of an acid chloride, opens up the carbonyl, closes it, kicks off the chloride. 06:13 And so, by this very simple, facile straight forward reaction, a whole variety of different penicillanic acid amine... amides could be synthesised and, of course, then tested for their efficacy. 06:31 One of those which you’ll undoubtedly be familiar with is amoxicillin, which is shown here, where the R group is that which is directly attached to an amine and then a hydroxybenzene. 06:43 So, let’s have a look at the structural components of these penicillins, shall we? Through all of these thousands of analogues that were synthesised and, obviously, tested for their antibiotic activity, it was discovered that the beta-lactam ring, the free carboxylic acid, the acylamino side chain and the bicyclic ring were all very useful in terms of activity. 07:08 However, only the beta-lactam ring itself was shown to be an absolute requirement for antibiotic activity. And this is shown in the blue circle there. 07:19 What this means is, if we lose that beta-lactam ring, we destroy the antibiotic activity. 07:25 Note also the cis-stereochemistry, the cis-relative stereochemistry, of the hydrogens on the alpha and beta carbons of that beta-lactam ring pointing backwards. This is quite important in terms of its recognition by the target enzyme.
About the Lecture
The lecture Introduction – Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Which structural part is important for the antibiotic activity of penicillin?
- Four-membered beta-lactam ring.
- Five-membered thiazolidine ring.
- Sulfur atom present in the thiazolidine ring.
- Total number of nitrogen atoms in the penicillin molecule.
- Total number of carbon atoms in the penicillin molecule.
Complete the following statement. β-lactam antibiotics are selective in their activity in human bodies because …
- … these drugs selectively disrupt the bacterial cell wall synthesis.
- … these drugs selectively disrupt the bacterial cell membrane synthesis.
- … these drugs selectively enhance the protein synthesis in human cells.
- … these drugs stimulate the synthesis of protective cell walls around the healthy human cells.
- … these drugs kill the infected human cells.
Which of the following is not a beta-lactam antibiotic?
- Levofloxacin
- Penicillins
- Cephalosporins
- Carbapenems
- Monobactams
Which of these is NOT a natural penicillin?
- Cefuroxime
- Benzylpenicillin (Penicillin G)
- Almecillin (Penicillin O)
- Phenoxymethylpenicillin (Penicillin V)
- Heptylpenicillin (Penicillin K)
Complete the following statement. For the production of semisynthetic penicillin, the starting precursor is …
- 6-aminopenicillanic acid (6-APA)
- Soya meal
- Yeast extract
- Whey
- Lactose
To carry out penicillin synthesis, which of the following fungi is used during the fermentation process?
- Penicillium chrysogenum
- Penicillium purpurogenum
- Penicillium roqueforti
- Penicillium stoloniferum
- Penicillium ulaiense
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |