Playlist
Show Playlist
Hide Playlist
Insulin Basics
-
Slides Endocrine Pancreas Endocrine System.pdf
-
Download Lecture Overview
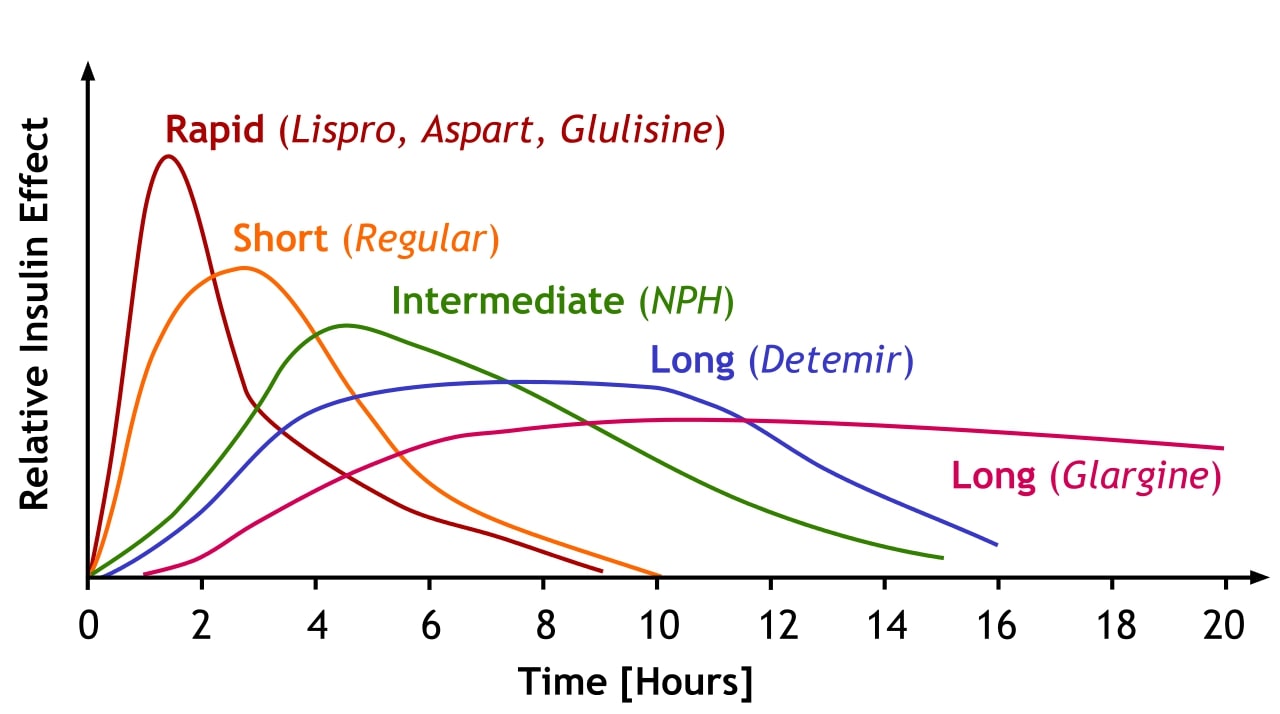
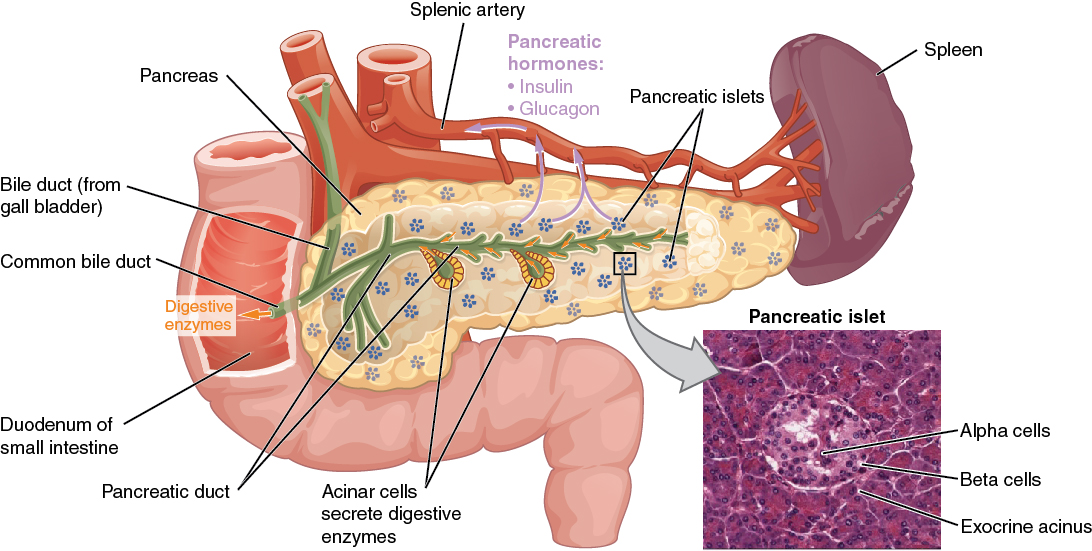
00:01 Let’s have a look at insulin synthesis. 00:04 Insulin is synthesized as a 110 amino acid long preproinsulin, composed of a signal peptide, depicted in orange, a B-chain in blue, the connecting peptide in yellow, which is a C-peptide to be exact and lastly the red part, which is the A-chain. 00:23 The signal peptide is later cleaved off, resulting in the formation of proinsulin. 00:29 Within the endoplasmic reticulum, three disulfide bonds are formed between cysteine residues. 00:36 Proinsulin is then transported to the Golgi apparatus, where the C-peptide is cleaved off. This process converts proinsulin into mature insulin, which consists of the B and A chains only. 00:51 Keep in mind that C-peptide is only produced during endogenous insulin production. Therefore, in the case of exogenous insulin, which is taken by diabetics, no C-peptide will be found in the blood. 01:06 Exogenous insulin is typically manufactured in recombinant DNA technology using Escherichia coli. 01:13 This type of insulin does not contain C-peptide, this allows the differentiation between true endogenous and false exogenously produced hypoglycemia. 01:25 Insulin. 01:28 Insulin forms usually of what glucagon is. 01:33 It's formed also in the islet and specifically in the beta cells. 01:38 It is also a peptide hormone similar to glucagon. 01:43 It is clear about 50% by the first path. 01:48 This is an important process because again it's going to mean that insulin is not around in the circulation very long. 01:56 In fact it's half life is only 3 to 8 minutes. 02:03 Importantly what we have produced at the same time as the peptide hormone insulin is something called C-peptide. 02:14 Don't get this mixed up with C reactive peptide. 02:17 This is just C-peptide. 02:20 C-peptide is when passed through this first pass system in a slower time frame. 02:29 Therefore, it has a longer half life. 02:32 About 35 minutes. 02:35 If you compare 35 minutes half life versus a 3 to 8 minute half life, you can see that C-peptide stays in the circulation longer. 02:45 How is that useful for you clinically. 02:47 How useful is that, you can use C-peptide if you measured in the blood as an index for how much insulin is being secreted. 02:59 Because insulin is not going to be around long enough to really measure it's concentrations. 03:04 But if you measure C-peptide, you know it's produced in a 1 to 1 ratio. 03:09 Therefore, if C-peptides goes up, insulin has to go up and vice versa. 03:16 You can see how this break down of this process works. 03:19 If you see this polypeptide being formed. 03:22 And there's a cleave that happens, that forms C-peptide and insulin. 03:28 So they'll be making this preformed proinsulin peptide. 03:34 You cleave a certain spot, leaving C-peptide and insulin as your two different secretory substrates.
About the Lecture
The lecture Insulin Basics by Thad Wilson, PhD is from the course Endocrine Physiology.
Included Quiz Questions
Plasma C-peptide can be used as an indirect measure of secretion of which pancreatic hormones?
- Insulin
- Glucagon
- Somatostatin
- Pancreatic polypeptide
What is the half-life of endogenous insulin?
- 3-8 min
- 15-20 min
- 20-45 min
- 12-14 min
- 16-19 min
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |