Playlist
Show Playlist
Hide Playlist
Hyperkalemia: Etiology
-
Slides Potassium Disorders Hypo and Hyperkalemia.pdf
-
Reference List Nephrology.pdf
-
Download Lecture Overview
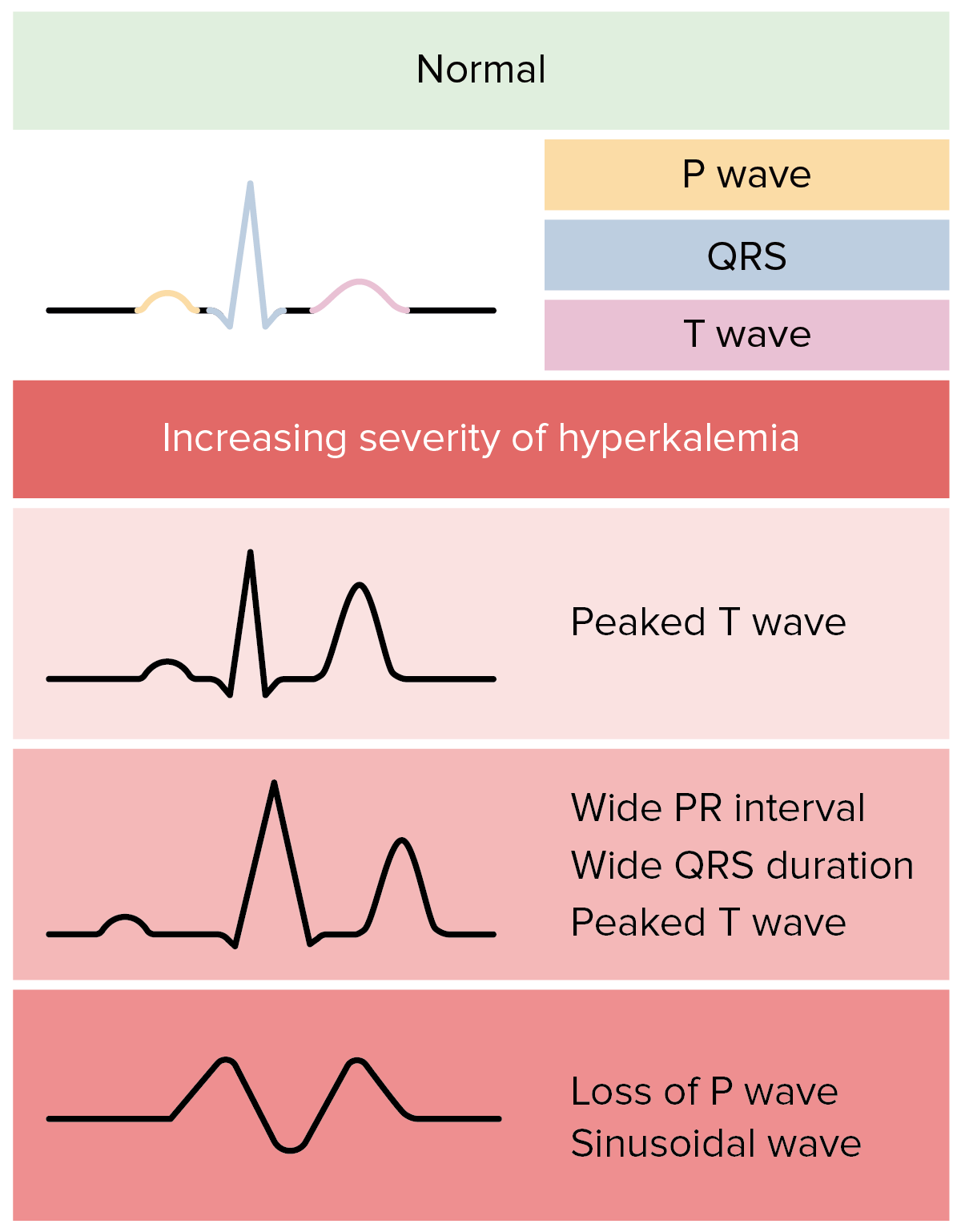
00:01 Let's move on to hyperkalemia. 00:03 Hyperkalemia is clinically defined by serum potassium greater than 5.2 milli equivalents per liter. 00:08 When we think about the ideologies we can group the mean either into pseudo hyperkalemia, transcellular shift, or we know mechanisms with decreased excretion. 00:17 Let's start with sudo hyperkalemia. 00:20 This occurs when there's a elevation in measured serum potassium due to potassium movement out of cells either during a blood draw or after a blood specimen has been drawn. 00:31 So it's not in vivo. 00:33 This could be because of hemolysis or destruction of red blood cells due to a technique during the blood draw if somebody's clenching or they have a prolonged tourniquet use during a venipuncture or you have venipuncture trauma that can cause hemolysis and release a potassium. 00:48 Thrombocytosis increased platelets can also cause e-flux of potassium once the blood has been drawn. 00:56 Same thing with leukocytosis. 00:57 So if I have a patient with acute leukemia who's coming in with an extraordinary high Y count I might see hyperkalemia. 01:05 However, this is really in vitro. 01:07 This does not happen in the patient and potassium is moving out of the cell. 01:11 Once that blood specimen has been drawn already. 01:16 Now we think about things like transcellular shift similar to hypokalemia there are things that can cause potassium e-flux from the cell to the extracellular fluid. 01:24 These are things like metabolic acidosis. 01:27 So think about what happens in acidosis. 01:29 Protons are going to enter the cell in order to buffer that extra cellular pth, but in order to maintain electroneutrality, we will have potassium enter the extracellular fluid in return. 01:40 This primarily applies to inorganic acids, not really organic acids like diabetic ketoacids. 01:45 It's so it really overall has a very small contribution or effect. 01:49 We have to think about hyperglycemia and hyperosmolarity. 01:53 So when somebody has an increase in elevation in serum osmolarity, think about what happens, water is going to move from the intracellular compartment to the extracellular compartment, and when that happens that results, in that results in an increase in intracellular potassium in the cell that high potassium will move out of the cell down its concentration gradient into the ECF. 02:14 So by solvent drag or from water movement out of the cell we end up with potassium e-flux seeing into that ECF. 02:20 Other mechanisms include non-selective beta antagonists. 02:24 These will interfere with potassium uptake into the cell by those beta adrenergic receptors. 02:30 Exercise also causes transcellular shift because potassium is released by muscle cells. 02:36 We need that because it causes local vasodilation for an increase in blood flow. 02:40 Tissue breakdown can also cause transcellular shift. 02:43 Things like Rhabdomyolysis, which is muscle breakdown or tumor lysis syndrome from a large tumor burden after chemotherapy and finally burns can all cause potassium release into that vascular circulation. 02:57 Digitalis or digoxin toxicity can inhibit that sodium potassium ATPase pump and that can lead to an increase in interest extracellular fluid potassium concentration. 03:07 And then finally there's hyperkalemic familial periodic paralysis. 03:11 This is kind of like a twin of the hypokalemic variant. 03:14 This is also an autosomal dominant process, but it's a point mutation in the skeletal muscle sodium channel rather than the dihydropyridine calcium channel. 03:21 And this is going to be precipitated by exactly the opposite things of hypokalemic periodic paralysis. 03:27 So this is going to be rest after exercise or having a large potassium ingestion. 03:33 So finally we come to our renal regulation of hyperkalemia, and this is due to a decrease in urinary excretion. 03:41 This can be due to either renal failure, volume depletion or functional hyperaldosteronism. 03:46 So in patients with renal failure, they're able to maintain near normal levels of potassium as long as that distal flow rate and aldosterone secretion is maintained. 03:55 Remember those were two of the four regulators that we talked about at that principal cell in terms of maintaining potassium balance. 04:02 So remember what L dosterone does again, I'm going to remind you one more time. 04:05 It's going to open those epithelial sodium channels, those renal outer medullary potassium channels and turn on that sodium potassium ATPase. 04:13 And that's really how potassium excretion is maintained. 04:17 Now hyperkalemia is going to develop in these particular patients, If they're oliguric, that means that they're not going to have that distal flow rate intact. 04:24 And if they have an additional problem as well like an excess potassium load or if they're on aldosterone blockade things like ACE inhibitors, angiotensin receptor blockers and aldosterone blockers. 04:36 We can also see hyperkalemia in states of volume depletion where we have a decrease in distal sodium delivery. 04:43 These are going to be states like hypovolemia or a decrease in effect of arterial blood volume, even though that patient may have total extracellular volume excess. 04:52 That's going to be a state like heart failure or cirrhosis of the liver. 04:55 Again, the mechanism here is that we have a decrease in distal sodium delivery in tubular flow rate. 05:02 Finally, we can have functional hyperaldosteronism either a low aldosterone state or resistance to the effect of aldosterone as a means of causing Hyperkalemia. 05:13 This can be due to either a mineralocorticoid deficiency in that could be primary adrenal insufficiency. 05:18 That means that the adrenal gland itself does not generate aldosterone from the Zona glomerulosa in response to renin. 05:26 This could be due to hyporeninemic hypoaldosteronism. 05:31 That is what we talk about as a diabetic or a type 4 renal tubular acidosis. 05:37 In any state that causes a low plasma renin level and a low aldosterone level. 05:42 We can also see this with tubular interstitial diseases things like sickle cell disease or urinary tract obstruction, which can affect the principal cell. 05:50 If we don't have that principle cell there or if it's not working properly remember that's the major site of potassium excretion. 05:56 And so if I don't have those epithelial sodium channels or renal outer medullary potassium channels to regulate my potassium excretion, I can end up with hyperkalemia. 06:06 And that is referred to as a distal hyperkalemic renal tubular acidosis. 06:10 Again, this is due to that impaired sodium reabsorption in the principal cell reducing both potassium and proton excretion. 06:17 There are drugs that can also mediate this. 06:19 So drugs that block the conversion to aldosterone or binding to aldosterone receptor. 06:25 These are things like a ACE inhibitors angiotensin receptor blockers, aldosterone antagonists like spironolactone or eplerenone. 06:33 We can have drugs that actually affect renin release. 06:36 So non-steroidal anti-inflammatories inhibit renin release beta blockers to a slight degree will also cause run an inhibition and then there's direct renin inhibitors like tekturna or aliskerin. 06:47 We have drugs that bind the luminol sodium potassium or luminal sodium channel that epithelial sodium channel at the principal cell. 06:55 Those are drugs like amilloride, triamterene, trimethoprim or pentamidine, all of those bind that ENaC Channel and because of that remember, that's going to abolish that electrochemical gradient. 07:06 So potassium will not favor, will not be favored to e-flux in the tubular fluid. 07:10 And then you can have drugs that cause multiple effects. 07:12 These are calcineurin inhibitors, drugs that we use for organ transplant to prevent rejection and they have an effect on both the epithelial sodium channel as well as that sodium potassium ATPase.
About the Lecture
The lecture Hyperkalemia: Etiology by Amy Sussman, MD is from the course Potassium Disorders: Hypo- and Hyperkalemia.
Included Quiz Questions
How does diabetic ketoacidosis cause hyperkalemia?
- Increased osmolarity
- Metabolic acidosis
- Tissue breakdown
- Decreased activity of Na/K ATPase
- Increased activity of K/H ATPase
Which of the following shifts potassium to the extracellular compartments?
- Digitalis
- Metabolic alkalosis
- Insulin
- Hyposmolarity
Which of the following is true regarding potassium urinary excretion?
- Aldosterone blockade increases the risk of hyperkalemia in patients with oliguric renal failure.
- Heart failure will cause volume overload, which will decrease serum potassium levels.
- Ifosfamide is associated with hyperkalemic renal tubular acidosis.
- Urinary tract obstruction impairs the outer medullary potassium channels in the principal cells, which will increase potassium excretion.
Which of the following pairings is correct?
- Sickle cell disease - functional hypoaldosteronism
- Amiloride - direct renin inhibitor
- Tumor lysis syndrome - pseudohyperkalemia
- Renal tubular acidosis type 1 - hyperkalemia
- Hyperkalemic periodic paralysis - dihydropyridine calcium channels
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |