Playlist
Show Playlist
Hide Playlist
Cysteine Metabolism
-
Slides AminoAcidMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
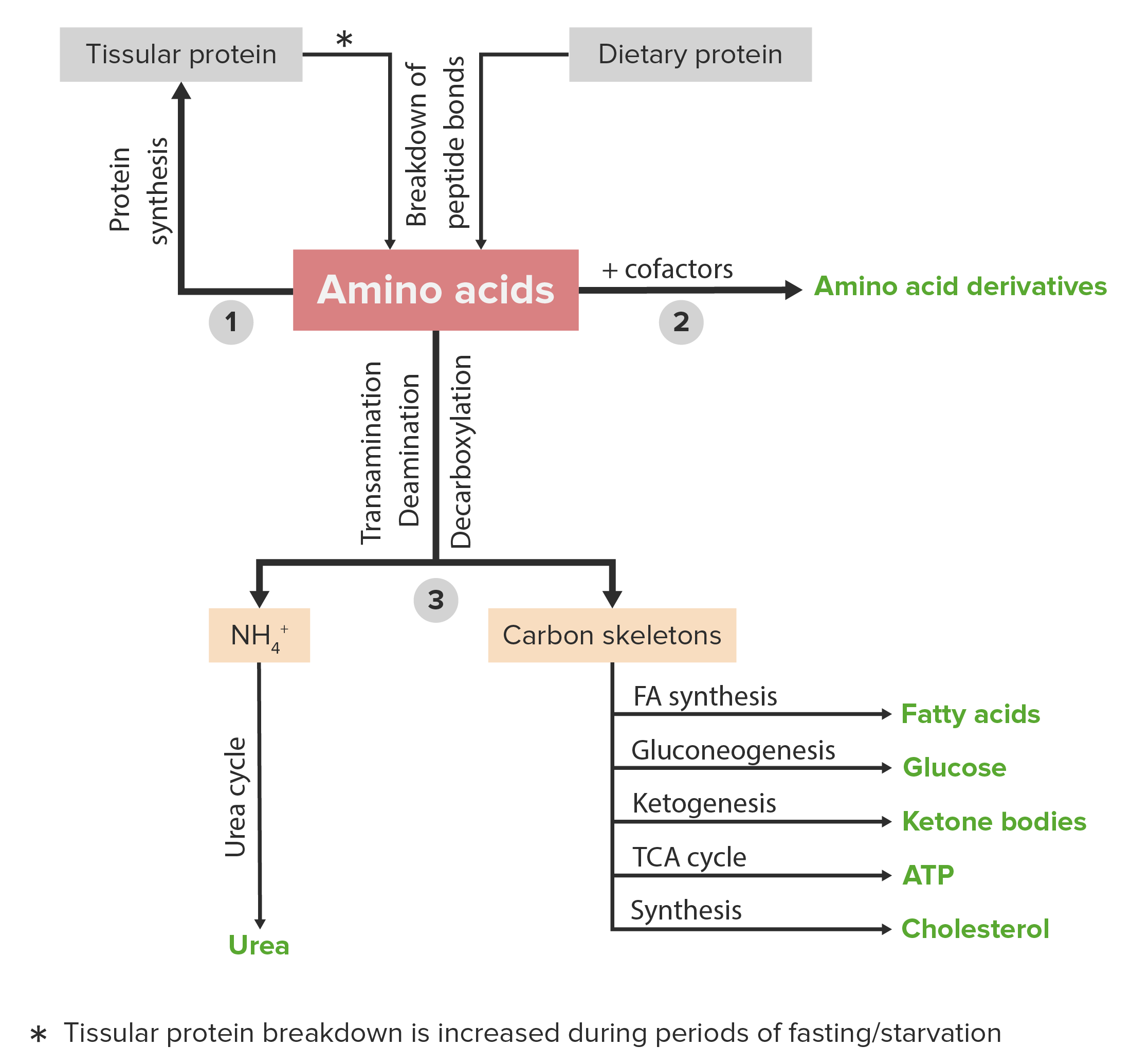
00:00 Now, homocystinuria, as I said, is a pretty severe disease. 00:05 It's a genetic disease caused by deficiency of this enzyme cystathionine beta-synthase. 00:11 There are many problems that can arise in homocystinuria. 00:15 There are musculoskeletal anomalies that occur. Intellectual disabilities. 00:19 And one of the reasons we have these intellectual disabilities is because of the problems with the nerve tissue in the brain. 00:26 Seizures can result. There are numerous eye anomalies that can arise. 00:30 Vascular disease, because again, we have a lot of homocysteine that's occurring because it's not being converted into cysteine in the process. 00:38 We see cystinuria, which is an unrelated genetic disease that has many symptoms that are similar. 00:45 We have the inability of the kidneys to reabsorb cysteine. 00:48 So, when this happens, then we see problems associated with the kidneys including high levels of cysteine, high levels of lysine, ornithine, and arginine that appear in the urine. 00:58 And because we have these things accumulating, we develop kidney stones that also occur. 01:03 This is not a disease that you would wish on anyone. 01:07 There are at least three other ways of making cysteine and I illustrate these here. 01:11 One of these comes from serine directly. 01:13 In this process, acetylcholine gets - donates an acetyl group to serine to make O-acetyl-L-serine. 01:22 That's shown here. And the next step of the process, the sulfur that ends up being in cysteine, comes with the addition of a hydrogen sulfide. 01:29 This splits out the acetate in the process, leaving behind cysteine for making proteins. 01:36 A second way of making cysteine actually comes from proteins itself. One of the things that cysteine can do in a protein is combine with another cysteine in a covalent bond to make this di-cysteine as were called L-cysteine. 01:49 This involves the joining of the two cysteines together by a disulfide bond shown in the middle of the molecule. 01:56 When proteins are cleaved and they've had this bond occur in them, L-cysteine is the product of the breakdown of the protein. 02:04 Making cysteine from L-cysteine is a trivial matter. 02:07 It simply involves breaking that bond and breaking-that bond involves a reduction. 02:11 The electrons in the process come from NADH, as you can see here. The product of the reaction producing two cysteines. 02:18 The last way of making cysteine starts with an oxidized form of cysteine known as cysteic acid. 02:25 Cysteine is fairly readily oxidized. 02:28 And so, it's not uncommon that cells will have L-cysteic acid within them. 02:33 To make cysteine from that simply involves a reduction, and that reduction involves, again, hydrogen sulfide, as we can see here. 02:41 In the process, the sulfur is donated to the cysteic acid. The sulfite, which was the oxidized sulfur, is released, and cysteine is produced. 02:50 Another member of the cysteine family is that of one of the rare amino acids I talked about earlier. 02:55 This amino acid is known as selenocysteine, and it does not normally occur in proteins. 03:00 It's very rarely appearing. 03:02 And its way of appearing in proteins is unusual and its synthesis is also a little unusual. 03:09 Selenocysteine is sometimes called the 21st amino acid because it's not coded for in the genetic code. 03:16 It uses a stop codon that's in the translational process to get into a protein, and I won't talk about that here. 03:25 The way in which selenocysteine is made is actually made on a transfer RNA. 03:30 Now, the other amino acid who has a modification that occurs on a transfer RNA is that of methionine, and I'll talk about that later in this set of lectures. 03:40 When we look at this process, we see what the way in which selenocysteine is made. 03:45 Selenocysteine synthesis starts with serine, which is why it's in the serine family. 03:50 But serine itself is not directly modified-until after the serine has been joined to a tran fer RNA. 03:57 You'll notice that this is a non-serine transfer RNA. 04:01 So, this is a special transfer RNA that has joined the serine in the process of making selenocysteine. 04:07 So, here we have the product of that reaction. The serine has been linked to this special transfer RNA. 04:14 There are two chemical reactions in which the serine is modified. 04:18 And the modification of the serine involves the alteration of the hydroxyl group to put a seleno group into that. 04:24 Two enzymes are involved known as SEL-A and SEL-D. 04:29 The product of that reaction is this selenocysteine linked to the transfer RNA. 04:34 And the selenocysteine linked to the transfer RNA is then incorporated into proteins using this odd stop codon mechanism of getting it in. 04:43 It's because of this that selenocysteine-is so rarely found in proteins.
About the Lecture
The lecture Cysteine Metabolism by Kevin Ahern, PhD is from the course Amino Acid Metabolism. It contains the following chapters:
- Cysteine Metabolism & Health
- Selenocysteine Metabolism
Included Quiz Questions
What are some of the clinical manifestations of homocystinuria? (Select all that apply).
- Accelerated atherosclerosis
- Gout
- Cirrhosis
- Cataracts
- Kidney stones
Which of the following statements is correct regarding selenocysteine? Select all that apply.
- It is not specified directly in the genetic code.
- It is not a true amino acid.
- It is synthesized from serine on tRNA.
Two cysteine molecules are produced by 1 chemical reaction from...?
- ...L-cystine.
- ...serine.
- ...L-cysteic acid.
- ...selenocysteine.
- ...homocysteine.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |