Playlist
Show Playlist
Hide Playlist
Congenital Heart Lesions – Congenital Heart Disease
-
9 Lecturio Congenital Heart Disease DLM v001.pdf
-
Reference List Cardiology.pdf
-
Download Lecture Overview
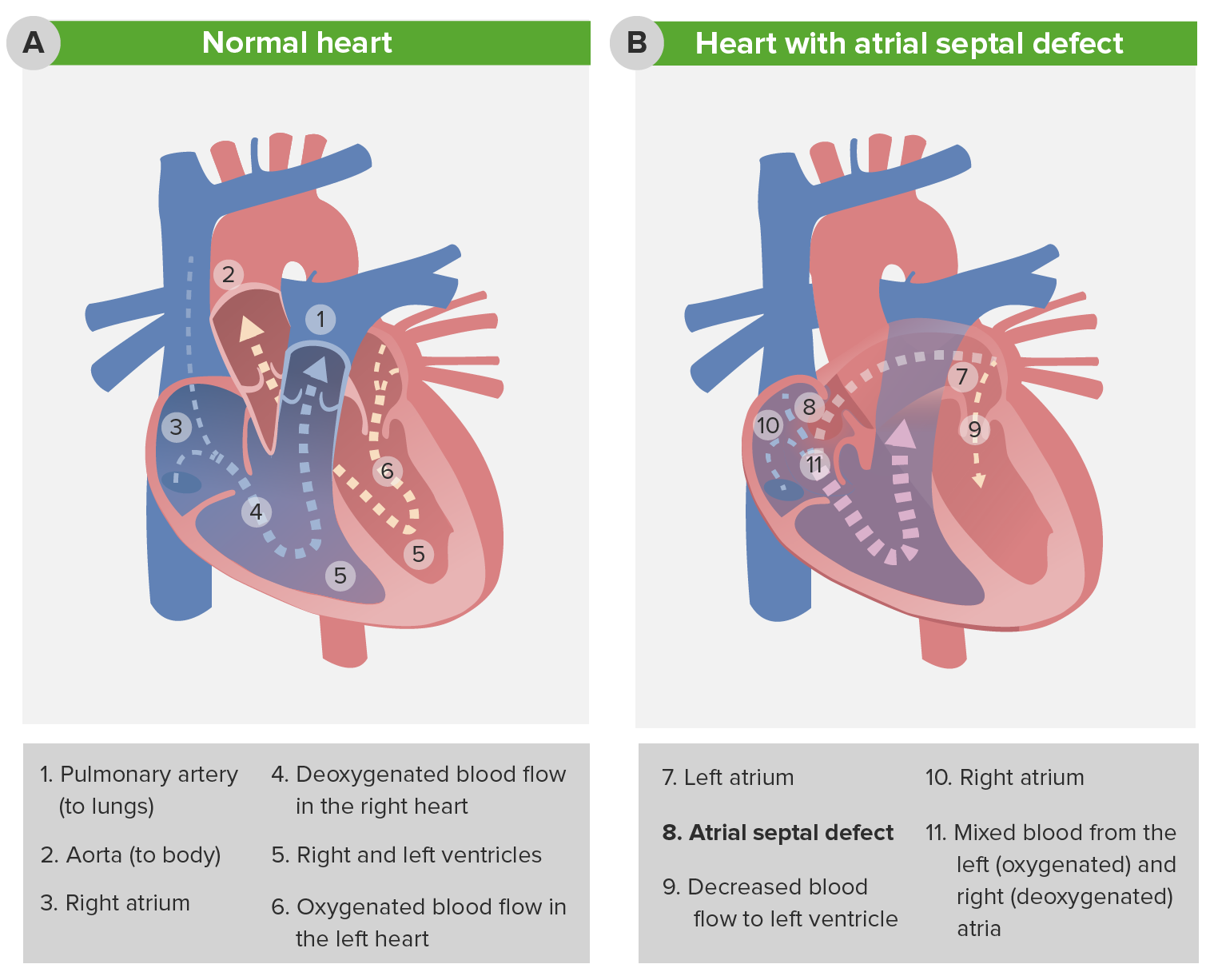
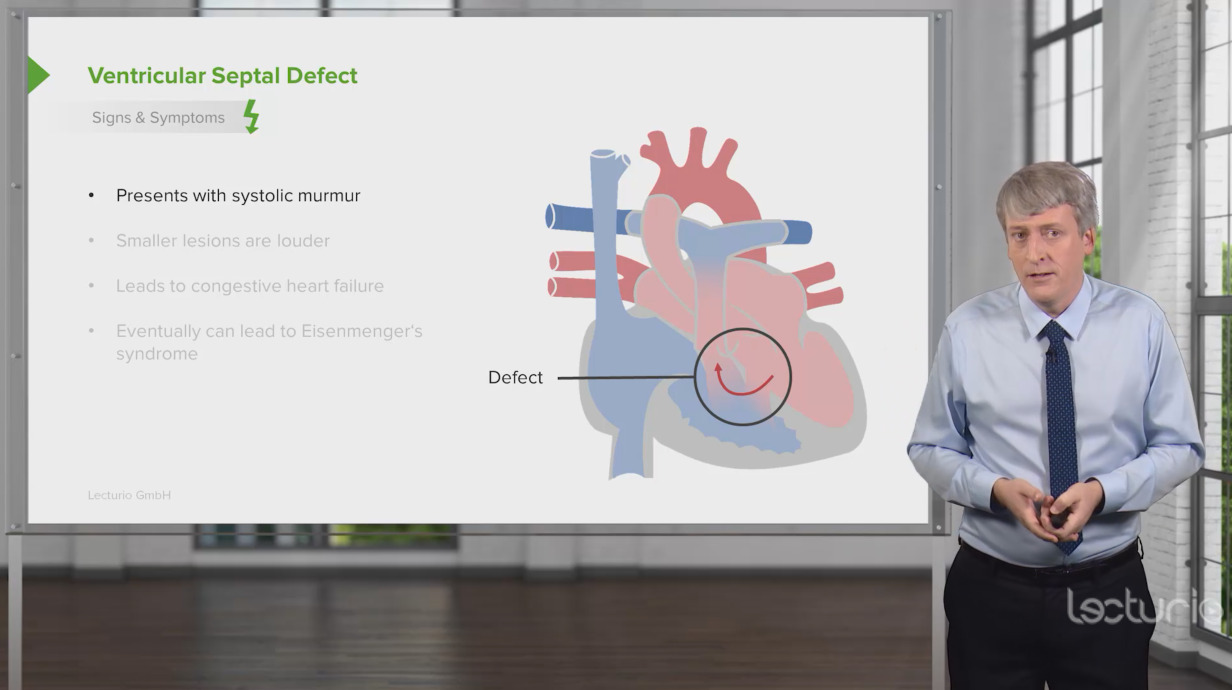
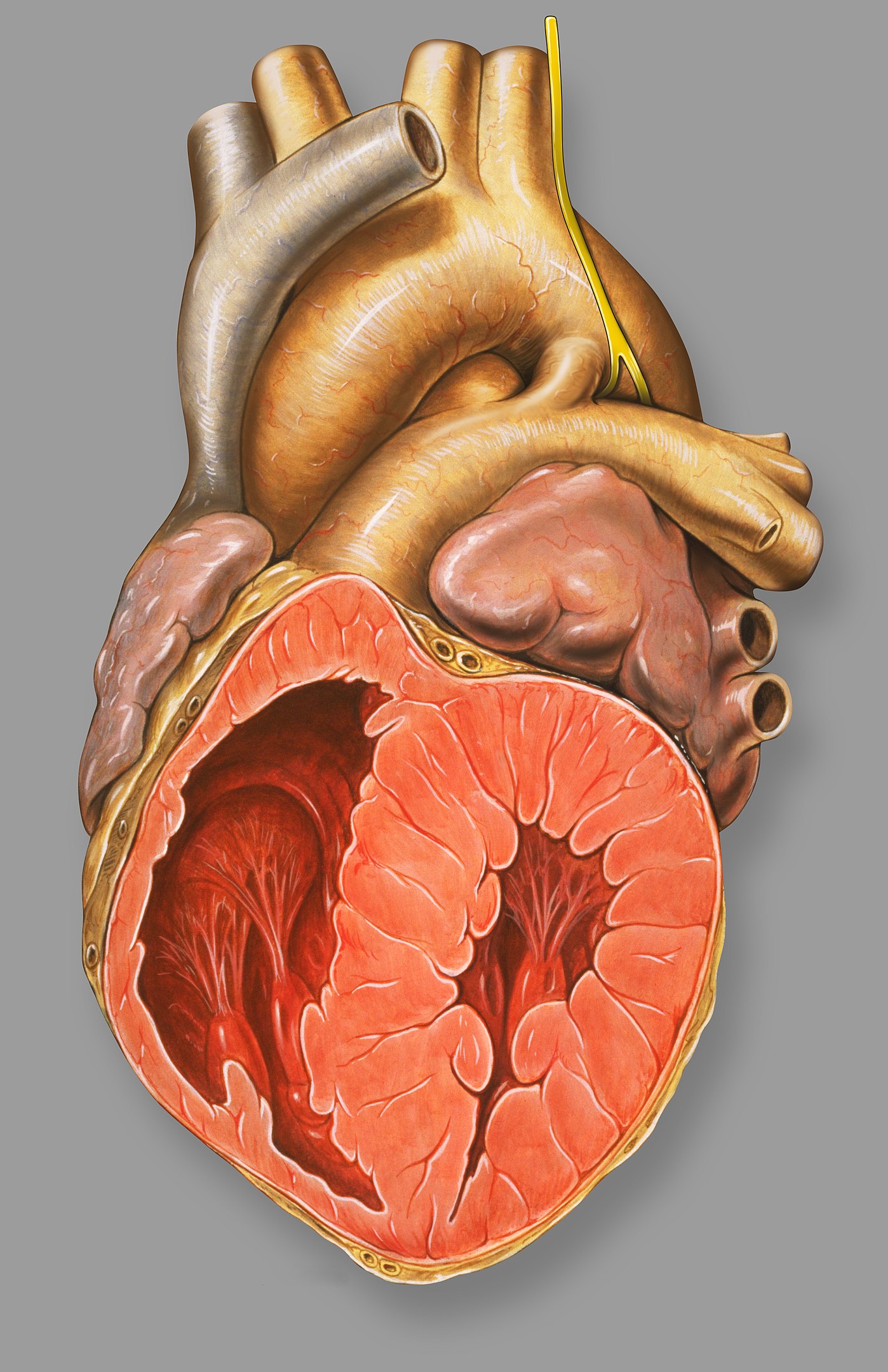
00:00 So, now, we’re going to deal with lesions where there’s a hole in the heart. These lesions are usually associated with so-called “left-to-right shunting of blood,” that is blood which is oxygenated from the left side of the heart ends up on the right side. 00:16 How does that happen? Let’s take atrial septal defect that I’m going to speak about in much greater length as an example. So, think about this - we’re talking about diastole, that is the heart is filling. The mitral valve is open, the tricuspid valve is open, blood is flowing allegedly into each ventricle. Oh, but there’s a hole in the septum! Well, it turns out, because the left ventricle is a little thicker than the right ventricle, it’s a little harder to fill the left ventricle than the right ventricle. So, in diastole, when all the valves are open, some of the red blood from the left atrium shunts across the defect in the atrial septum and into the right ventricle. One of the ways we diagnose it is we find there’s too much oxygenated blood in the right… in a sample taken from the right ventricle. And often, as much as couple of liters a minute may shunt across the left atrial… from the left atrial area into the right atrial area through the defect and lead to marked increase in the amount of blood that the right ventricle has to pump. 01:23 In a ventricular septal defect, the same thing happens. Left ventricular pressure is much higher than right ventricular pressure. When the left ventricle squeezes and the right ventricle squeeze together, the pressure’s higher in the left ventricle. So, red blood shunts over into the right ventricle. With a patent ductus arteriosus, that’s the connection between aorta and pulmonary artery from embryonic life. It doesn’t… supposed to close down, but it doesn’t close down. Aortic pressure is higher than pulmonary artery pressure. 01:53 So, throughout systole and diastole, there’s red blood shunting into the pulmonary artery. 02:00 You can see, in each of these conditions, extra red blood is arriving in the pulmonary circuit. In ASD and VSD, it’s arriving in the ventricle, the atria and the ventricle. 02:12 And in PDA, it’s arriving at the level of the aorta or the pulmonary artery. And what this means is there’s increased pulmonary blood flow and if this goes on for a period of time, you can have increased pulmonary resistance, severe pulmonary hypertension and the shunt can actually reverse and go right to left with blue blood ending up in the arterial circulation. Patients have blue lips, they’re markedly disabled often because they’re not getting normal oxygenation of the red blood in their arterial circulation. 02:50 So, let’s talk for a few minutes about atrial septal defect. It’s the commonest lesion that slips through pediatric years and into adult life. It usually requires closure. These days, closure is sometimes done in the catheterization laboratory with a special kind of catheter that puts a little… little umbrella on the defect and closes it. Although occasionally, if it’s large, it requires surgery. In many instances, it’s already been corrected in childhood and there are some few minor problems that can occur later on in life, some arrhythmias and so forth, but most of these patients do perfectly well and lead normal life expectancies if the defect has been closed. If the defect isn’t closed, as I mentioned before, the increased pulmonary blood flow can lead to pulmonary hypertension and eventually, right ventricular failure, cyanosis, all kinds of things that markedly disable a patient. The hole is in the atrial septum and in embryonic life, often there’s a hole there because you want to shunt blood from right, oxygenated blood from the placenta from right to left, but it’s supposed… that hole is supposed to close in very early life after the baby is born. Now, you can see here, from this diagram, what happens to the heart. You get an enlarged right ventricle because there’s left-to-right shunting. Remember, we talked about that, the reason because the right ventricle is a little more flexible compared to the left ventricle. So, some of the red blood to the left atrium ends up in the right ventricle and the result is that there’s very much higher oxygenation in the right ventricle and the pulmonary artery than normally would be there. Because normally, remember, the blood coming back to the right ventricle and to the right atrium is the blood that’s had its oxygen removed by in the capillary phase and passed in the veins up to the… up to the right atrium and right ventricle. 04:52 One of the findings, if the patient goes on to severe pulmonary hypertension, in other words, the lesion is not fixed, one of the lesions that occurs, of course, that makes the diagnosis very easy is what you see here in this picture. This is known as “clubbing”. 05:08 It’s abnormal rounding of the fingernails. This is a very advanced example and by the way, if you look, the fingernails are not the normal color, they’re not the normal pink color. They’re a darker color and that’s because you have blue blood in the arterial circulation. The pulmonary hypertension has become so severe that now, instead of the red blood shunting across into the right atrium and down into the right ventricle, in fact, blue blood is shunting across the septum into the left atrium and the left ventricle and being pumped out in the aorta. This is commonly known as “Eisenmenger syndrome.” It’s because of severe increase in pulmonary vascular resistance from longstanding, uncorrected shunting. It occurs more commonly with ventricular septal defect when higher pressures are seen in the right ventricle than occur in atrial septal defect. Nevertheless, this is a very dreaded complication of congenital heart disease. And, just here, again, so you see, here’s a little diagram of the heart just to tell you what the findings are in a patient with atrial septal defect, why it’s so subtle. There’s often a small systolic murmur from the pulmonic valve, but nowhere near as remarkable as with pulmonic stenosis. You may actually, by putting your hand on the chest, feel the right ventricle, which you normally wouldn’t feel in somebody and the second heart sound is wide and stays wide. Now remember, with respiration, we talked about this early on when we talked about the normal cardiac exam, the second heart sound splitting moves with respiration. It gets wider during inspiration and narrower during expiration. Let me show you an example: It’s, “BUM-pa-dum, BUM-pa-dum.” That’s in inspiration, and in expiration, it’s, “bum-pa-DUM, bum-pa-DUM, bum-pa-DUM.” So, it gets narrower. What happens in atrial septal defect because the right ventricle is overfilled, that movement of the splitting of the second heart sound doesn’t occur, so you hear, “LUB-pa-dum, LUB-pa-dum, LUB-pa-dum.” You never hear that sound narrowing. And then there’s findings on the electrocardiogram and of course, on the echo, that confirm your diagnosis. But, those findings are pretty subtle, and particularly in a little child, where the heart’s going, “BUDDA-BUDDA-BUDDA-BUDDA-BUDDA-BUDDA,” it’s very easy to miss that fixed… so-called “fixed” splitting. So, here we see a little diagram, again, of one of the lesions that can increase the pulmonary blood flow, for example, ASD and you can see it accounts for 7% - 8% of congenital heart disease, which is the largest fraction. It’s quite common as... discovered lesion in adults, whereas usually the VSD because it creates a loud systolic murmur, is usually picked up in childhood. Interestingly enough, VSD can spontaneously close during childhood because of some hypertrophy of the septum. So, often, pediatricians will follow smaller VSDs; they don’t have to be closed. Larger VSDs, of course, create a large pulmonary blood flow and do require closure. It turns out that ASD’s a little more common in males than in females, and as you see, about 7% to 10% of congenital heart disease is an atrial septal defect. There are several forms of atrial septal defect depending upon where in the septum the hole occurs. The common one is called an “ostium secundum.” It’s the one that’s right over the foramen ovale, that’s the little opening in atrial life that never closes or doesn’t close completely or can be, actually, a failure of the atrial septum to develop normally. It’s right in the center of the atrial septum and I’m going to show you a picture of it. And here’s an operative picture. You can see the surgeon’s instruments to the left and you can see, we’re looking at the atrial septum and it’s quite clear that you’re looking at a hole. And what you’re seeing in the… on the other side is the left atrium. So, you can see, here’s a defect. This is right in the area where the foramen ovale would have been. It’s also much larger. So, this was a failure of the atrial septum to develop normally. And here’s the operative picture. Usually a little piece of pericardium that is the lining around the heart is taken or you can use a Dacron or a Teflon patch and the defect is closed. So, this is a successful closure of an atrial septal defect. This actually looks… the heart looks reasonably large, although this is a magnified picture, but I’ll bet this was not in a tiny infant. This was probably in an adolescent or possibly an adult. 10:01 We’re going to spend a little less time on the other lesions, but ventricular septal defect is the commonest lesion in childhood, but becomes much less common in adult life for a couple of reasons. First of all, as I mentioned before, the ventricular septum often enlarges and closes the defect spontaneously. And secondly, because the murmur is loud and easily heard in childhood, often it’s repaired, either with a catheter patch that’s put in or with surgery. And so, the ventricular septal defect ends up, in adult life, to be much less common than it is in childhood. Number two in childhood is ASD, which becomes the most common in adult life because it can be missed because the findings are subtle. 10:45 Again, you have increased pulmonary blood flow because during systole, the left ventricle is pushing red blood into the right ventricle. It goes out in the pulmonary circulation. 10:56 Again, the most dreaded complication is marked increase in pulmonary vascular resistance; the Eisenmenger syndrome in which the patient is cyanotic - they’re blue. They have decreased cardiac output. They are markedly disabled. Rarely, they will survive to late middle life. 11:15 Usually, complications occur that cause death sometime, 40s or 50s. The patent ductus arteriosus is the third increased blood flow… pulmonary blood flow lesion. Again, the ductus arteriosus is used in embryonic life. It’s supposed to close at the time of birth. If it fails to close and stays open, then again, aortic pressure being higher than pulmonary artery pressure results in a left-to-right shunt that is red blood into the pulmonary artery, markedly increasing pulmonary blood flow and also, by the way, increasing pulmonary pressure because aortic pressure is being transmitted to the pulmonary artery. These individuals more rapidly develop Eisenmenger syndrome, that is severe pulmonary vascular disease, severe constriction in the pulmonary small vasculature that leads to pulmonary hypertension, right ventricular failure, blue blood shunting across the PDA into the aorta, and again, the Eisenmenger syndrome, marked disability and early mortality. This murmur… this murmur associated with a PDA is one of the most interesting ones in cardiology. Why? Because aortic pressure is always higher than pulmonary pressure. It’s higher during systole and it’s higher during diastole. So, that means you have shunt flow both during systole and diastole and turbulent flow in the pulmonary artery. So, when you listen along the left upper border of the sternum here, what do you hear? You hear a continuous murmur that’s a little louder in systole (sound). It’s a murmur that’s very, very distinctive. 12:58 When you hear it, you know immediately what you’re dealing with. You’re dealing with a continuous flow, both in systole and diastole, continuous flow of blood flow, red blood from the aorta into the pulmonary artery. This is closed, by the way, with an operation where you don’t need the heart–lung machine. Why? Because you’re not getting inside the heart. You’re just tying off and dividing and separating the two halves of the patent ductus arteriosus. It was the first congenital heart lesion corrected by surgery because of course, it was corrected before the heart–lung machine was available. 13:33 And again, as I mentioned, the hemodynamics of it are both increased flow and increased pressure in the pulmonary circuit that can lead to severe pulmonary vascular disease. 13:45 But fortunately, the characteristic murmur means it’s usually picked up in childhood and corrected with a fairly simple cardiac… cardiovascular operation that does not require the heart–lung machine. And again, I mentioned the findings. You’re going to hear a continuous murmur. You may… the patient may have bounding peripheral pulses because there’s a runoff from the aorta into the pulmonary artery. In a sense, there’s a decrease in resistance to… in the aorta, and consequently, this can lead to a very marked brisk pulse. There’s a widened pulse pressure because of that when you take the blood pressure. So, there’s a number of findings, and again, it’s almost always picked up in childhood because of these findings and it is corrected in childhood. 14:37 Treatment of patent ductus arteriosus consists of interrupting the ductus. This can be done with a catheter, with a little plug over a wire or it can require operative treatment, particularly if it’s big and it looks like it’s going to be more complicated. It can be closed with a surgical procedure where one opens the chest and ties it off and interrupts it, cutting it after it’s been tied off on both sides. These days, most of these are closed in the catheterization laboratory, but a small number are a little more complex and require surgical intervention. The final form of congenital heart disease, the cyanotic form, the ones with the ventricles switched and the aorta or pulmonary artery switched and so forth are very complex. They are seen immediately in childhood because the baby is blue - the baby is cyanotic. They always require urgent surgical intervention and usually, what we see in adult life is not a cyanotic patient, but what we see is somebody who’s already had one or multiple surgical procedures. They are very complex. 15:40 We actually don’t even know yet what the full, natural history of these lesions are going to be because they’ve only been operated on for 30 or 40 years. And so, the full life expectancy of what happens after repair of so-called “transposition of the great arteries” where the pulmonary artery and the aorta are switched on the two ventricles is not fully known. And also, a number of different operations have been done, so we don’t know exactly what’s the long-term outcome of Operation A versus B. Again, these individuals are always followed by pediatric cardiologists, usually alongside an adult cardiologist who specializes in congenital heart disease in the adult. So, these folks often fall into the category of partially repaired or palliated congenital heart disease because you can’t completely change around the anatomy, which is so abnormal. That’s different from the ASDs, the VSDs, pulmonic stenosis and aortic stenosis. All of those previous ones, the patients can get to near-normal life or normal life expectancy and normal activity. These lesions, the ones with cyanotic forms, are much more complicated and often, the operations don’t completely change the environment; the patients live on with significant disability.
About the Lecture
The lecture Congenital Heart Lesions – Congenital Heart Disease by Joseph Alpert, MD is from the course Cardiac Diseases.
Included Quiz Questions
Which one of the listed conditions leads to left to right shunting?
- Patent ductus arteriosus
- Pulmonary stenosis
- Atherosclerotic heart disease
- Coarctation of the aorta
- Aortic stenosis
Which of the following diseases does NOT cause clubbing of the fingers?
- Atrial fibrillation
- Total anomalous pulmonary venous return
- Transposition of the great arteries
- Truncus arteriosus
- Tetralogy of fallot
Which of the following is the most common type of atrial septal defect?
- Ostium secundum
- Sinus venosus
- Ostium primum
- Coronary sinus
- Atrioventricular canal defect
Typically, the murmur in patent ductus arteriosus is loudest at which of the following locations?
- Upper left border of sternum
- Upper right border of sternum
- Over the Back
- Lower right border of sternum
- Upper right clavicle
Classic continuous machinery murmur is typically heard in which of the following conditions?
- Patent ductus arteriosus
- Aortic stenosis
- Ventricular septal defect
- Coarctation of aorta
- Atrial septal defect
Which of the following is NOT a typical feature of left to right shunts?
- Cyanosis
- Minimal symptoms
- Tolerated for decades
- Eisenmenger syndrome if large and severe enough
Ventricular septal defect has which of the following types of murmurs?
- Pansystolic murmur
- Still’s murmur
- Mid diastolic murmur
- Early diastolic murmur
- Ejection systolic murmur
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
As always, very helpful. Great examples. Enhanced my understanding on the topic without information inundation.