Playlist
Show Playlist
Hide Playlist
Blood Gas Transport Comparison – Gas Transport
-
Slides Gas Transport.pdf
-
Download Lecture Overview
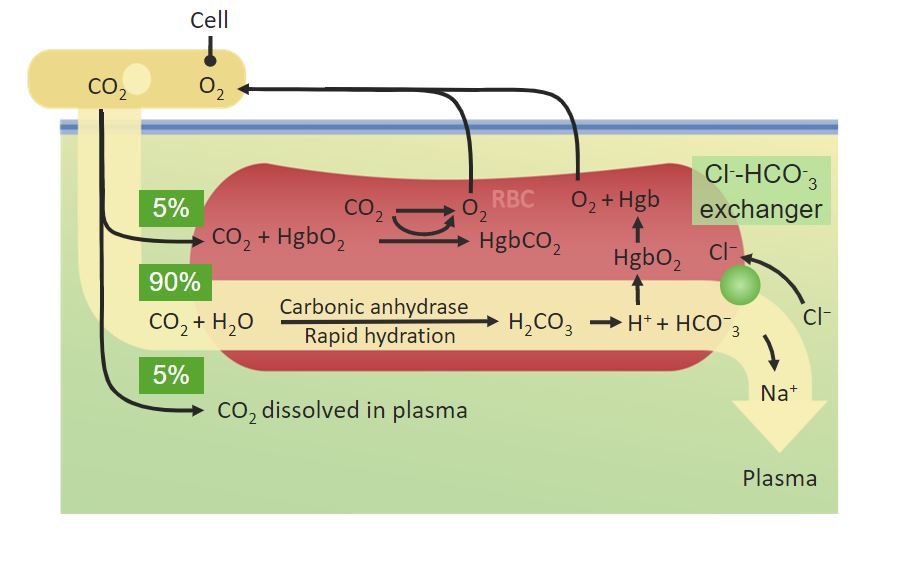
00:01 Okay. 00:02 As we transition now to carbon dioxide transport, we’re going to use a compare and contrast point of view to get us started. 00:12 Since we’ve already covered the O2, I know you know this now, and what we’re going to do is add the CO2 to that so that you can start to understand CO2 and then we’ll finish up with the gas transport. 00:26 So the first aspect is that CO2 more in the arterial side of the circulation is dissolved than what is for O2. 00:37 Now, the partial pressure of CO2 on the arterial side of the circulation is lower. 00:42 So this must mean that carbon dioxide solubility is greater than oxygen solubility. 00:50 Also, less of carbon dioxide is bound to hemoglobin. 00:56 So there must be a different way in which it’s bound to hemoglobin molecule itself, where it has a low amount of affinity, compared to oxygen, which is 98% bound to hemoglobin. 01:08 The final thing that’s different with carbon dioxide transport is most of it, 90%, is in the form of bicarbonate. 01:17 So bicarbonate is part of a reaction in which you can take CO2, add it to water, it can form carbonic acid and then dissociate into a bicarb ion and a hydrogen ion. 01:30 And this is this bicarb that is in the form of carried CO2. 01:35 So you always think of CO2 as a gas, but in this case, CO2 can be in ion as well. 01:41 And that’s how your body transports the majority of the carbon dioxide within the blood, is in the form of an ion, not in the form of gas. 01:50 Okay. 01:51 Let’s go through each of these mechanisms one by one.
About the Lecture
The lecture Blood Gas Transport Comparison – Gas Transport by Thad Wilson, PhD is from the course Respiratory Physiology.
Included Quiz Questions
Which of the following statements is NOT true regarding carbon dioxide transport in the blood?
- The solubility of oxygen is greater than carbon dioxide.
- There is more CO2 dissolved in the blood than O2.
- Less carbon dioxide is bound to hemoglobin as compared to oxygen.
- The majority of the carbon dioxide in the body is in the form of bicarbonate ions.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |