Playlist
Show Playlist
Hide Playlist
Bbb Penetration – Pharmacokinetics and Pharmacodynamics
-
Slides 12 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
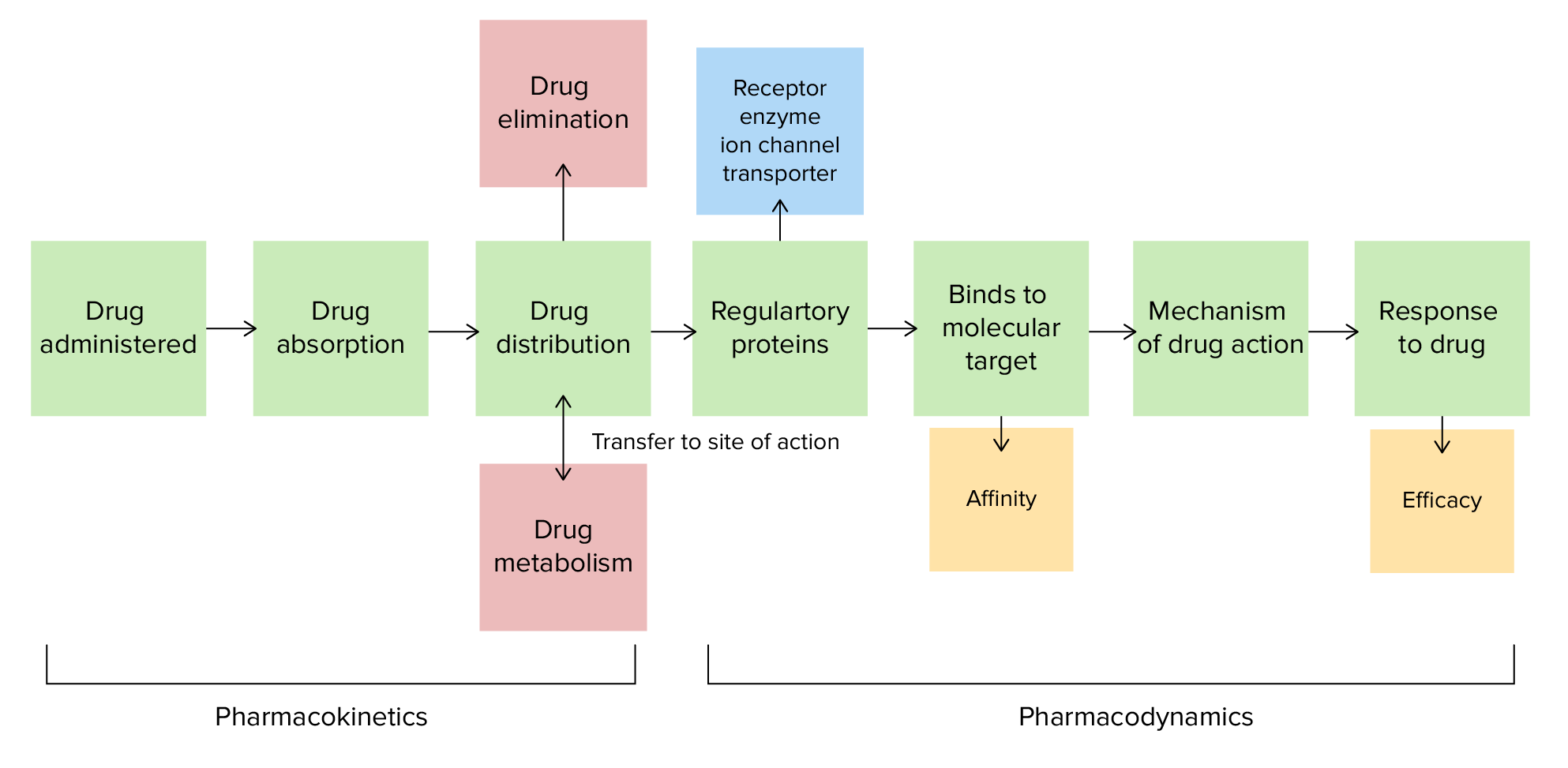
00:01 And this is an example of where you can overcome this issue by changing the structure of the molecule. You are undoubtedly familiar with codeine. Codeine is a painkiller that’s commonly administered with paracetamol and it’s closely related to morphine. Now, morphine, as you know, is a very, very strong analgesic. It binds tightly to Mu (μ)-opioid receptors. How it’s not nearly as able to pass through the Blood Brain Barrier as diamorphine, otherwise known as heroin. And what you can do here, as you can see, is if you acetylate those morphine OH groups which are polar and actually inhibit passage through the Blood Brain Barrier, you create something which is by far more lipophilic. It is a low molecular weight and therefore, can pass easily through the Blood Brain Barrier where all that happens is it’s hydrolysed in the 3 position as you can see here as indicated. Hydrolysis gives rise to that 3 OH group which then interacts directly with the Mu (μ)-opioid receptor and is a crucial path for the pharmacophore for that analgesic activity. Codeine is also, as you might expect, an analgesic, but is no way near as potent as morphine or diamorphine, not least because it exists as an ether. Note, you have a methyl group attached to an oxygen attached to the benzene ring at 3 position. Ether groups are very difficult to remove. There is such a thing as a Catechol-O-methyltransferase, which we may touch on in the next lecture, but the reality here is that only a small amount of that methyl group is hydrolysed to give you the free alcohol, which is a crucial part of the pharmacophore for binding at the Mu (μ)-opioid receptor. 01:49 This results, as you can clearly see, in a substantial variation in the bioavailability of the drug. By masking a polar group with a non-polar one, it’s possible to improve the passage of a drug through the Blood Brain Barrier. 02:05 So, let’s summarise. We have a problem with the issue of permeability. And where we have that from a pharmacokinetic perspective, we can increase lipophilicity, we can reduce polarity and the propensity for ionisation and we can consider prodrug approaches by temporarily masking those groups that are causing these problems. 02:27 When we are talking about solubility, we can either add an ionisable center or increase the polarity of the molecule by adding the potential for hydrogen bonding, for example, incorporating a carboxylic acid, amine or water molecule. We can decrease the lipophilicity by abstracting or removing those groups that make it more lipophilic, groups like benzene, cyclohexyl, indeed, any long chain aliphatic group. And finally, to improve the bioavailability in the… through the Blood Brain Barrier into the brain, increase lipophilicity and also, as we’ll see again, prodrug approaches. Thank you.
About the Lecture
The lecture Bbb Penetration – Pharmacokinetics and Pharmacodynamics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
The acetylation of the hydroxyl groups of codeine does which of the following?
- It masks the polar nature and enhances the BBB crossing capabilities of codeine.
- It masks the lipophilic nature and enhances the BBB crossing capabilities of codeine.
- It exposes the polar nature and enhances the BBB crossing capabilities of codeine.
- It masks the lipophilic nature and suppresses the BBB crossing capabilities of codeine.
- It masks the neutral nature and enhances the BBB crossing capabilities of codeine.
Customer reviews
1,4 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
2 |
| 1 Star |
|
12 |
15 customer reviews without text
15 user review without text