Playlist
Show Playlist
Hide Playlist
Aromatic Family: Phenylalanine and Tyrosine
-
Slides AminoAcidMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
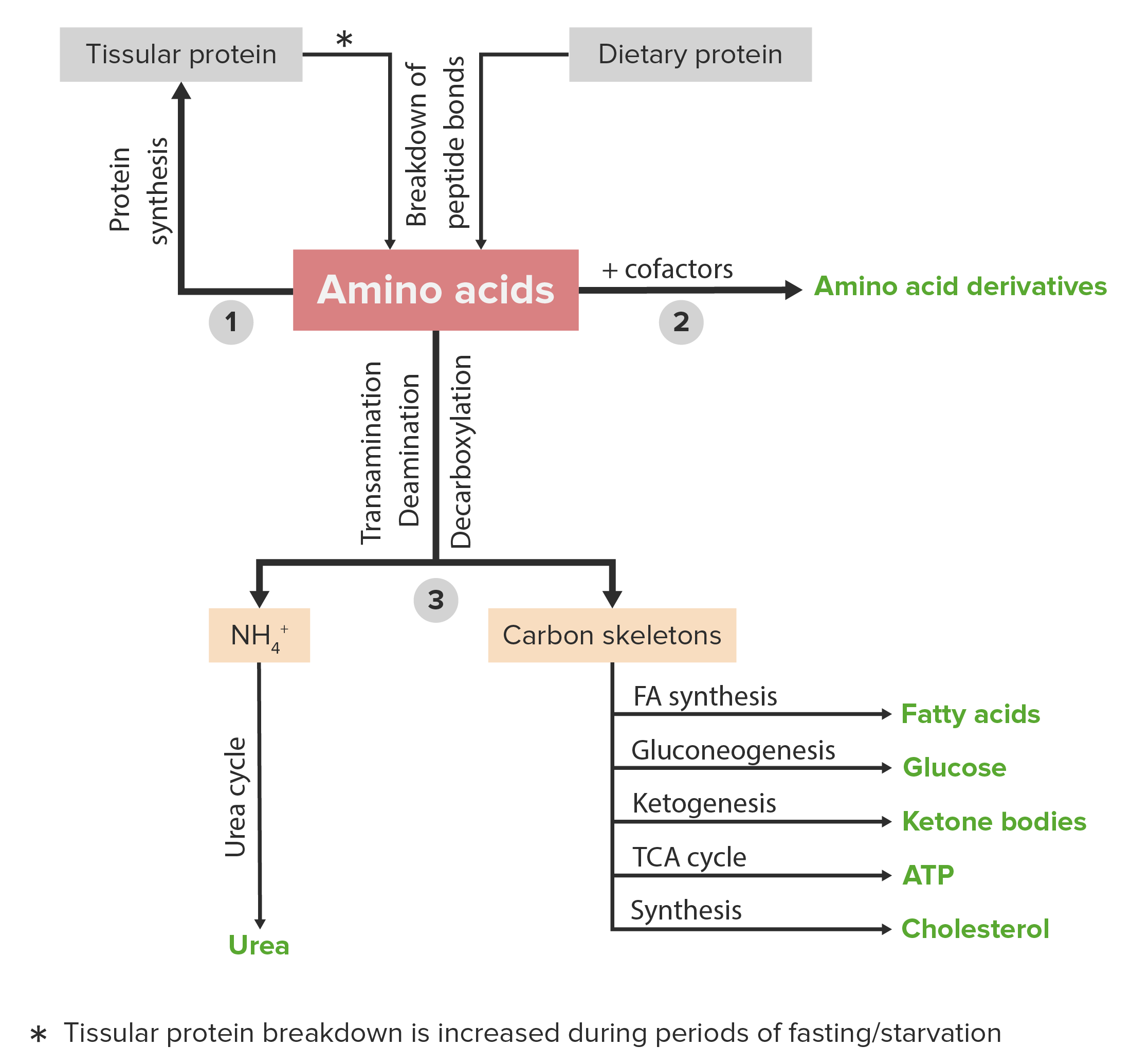
00:01 So phenylalanine is an essential amino acid. 00:04 That means, of course, it has to be in our diet. 00:06 It is also a precursor of the amino acid tyrosine. 00:10 Now phenylalanine metabolism requires the enzyme phenylalanine hydroxylase. 00:15 That catalyzes the formation of tyrosine form phenylalanine in the reaction that we can see here on the right. 00:22 In this reaction, phenylalanine is getting a hydroxyl group. 00:25 And this is a complicated hydroxylation reaction that involves tetrahydrobiopterin and molecular oxygen. 00:32 The products of that reaction being dihydrobiopterin and water, plus the amino acid phenylalanine and you can see the different between the phenylalanine and the tyrosine is the addition of that hydroxyl group. 00:44 A deficiency of this enzyme, phenylalanine hydroxylase, is very, very important because this enzyme when it's deficient causes the disease known as phenylketonuria. 00:56 Fortunately, phenylketonuria can be treated if it's diagnosed early and it's commonly diagnosed at birth. 01:02 If it's not diagnosed and not recognized early enough, then high levels of phenylalanine in the diet damage the brain and you get sever neurological consequences. 01:12 Now fortunately, as I said, it's treatable by reducing the amount of phenylalanine that a person gets in their diet, and this works fairly well. 01:19 But one of the complicating factors is that the artificial sweetener, Nutrasweet, contains phenylalanine. 01:27 So a person who has phenylketonuria should absolutely not consume Nutrasweet, and that's why you see the warning on cans of soda, for example, that contain Nutrasweet. 01:39 Tyrosine is an amino acid whose essentialness or not essentialness depends upon whether or not phenylalanine is present. 01:46 If it's present in the diet, then tyrosine is not essential because tyrosine can be made from phenylalanine. 01:52 Tyrosine is important as a precursor of catecholamines. 01:56 So just as tyrosine was a product of phenylalanine breakdown, the catecholamines are a product of tyrosine breakdown. 02:04 There are four important catecholamines that are made from tyrosine: L-DOPA, L-dopamine, norepinephrine, and epinephrine. 02:12 These molecules are important hormones and neurotransmitters that function in our brain. 02:19 Tyrosine also is important because it is a source of electrons to reduce chlorophyll in photosystem II during the process of photosynthesis. 02:28 Tyrosine forms the radical that's essential for the function of ribonucleotide reductase which I will discuss in the lectures on nucleotide metabolism. 02:39 Now tyrosine's synthesis of the four catecholamines is shown in this slide. 02:44 As I said, this is actually the breakdown of tyrosine that produces this. 02:48 We see the first product being made from tyrosine here using tyrosine hydroxylase and again, tetrahydrobiopterin and molecular oxygen, producing dihydrobiopterin and water and this compound called L-DOPA. 03:02 We can see that L-DOPA defers from tyrosine by the addition of a single hydroxyl group. 03:08 L-DOPA is converted into L-dopamine by the enzyme aromatic acid decarboxylase, and as its name suggests, this involves lost of a carboxyl group which is occurring on the left portion of the molecule and that's where we see the difference between the L-dopamine and L-DOPA. 03:26 L-dopamine itself then can be converted into norepinephrine by a reaction involving vitamin C, this is where vitamin C is important, molecular oxygen. 03:36 The enzyme catalyzing this is dopamine beta-hydroxylse. 03:40 And we can see in this reaction that there's another hydroxylation that's occurring, the hydroxyl group being added on the top part of the molecule near the left. 03:49 In the last of these reactions, L-norepinephrine is converted into L-epinephrine. 03:54 This reaction is a methylation that occurs and that methyl group is donated by the molecule S-adenosylmethionine to produce S-adenosylhomocysteine. 04:03 The reaction is catalyzed by the enzyme whose name you can see on the right. 04:08 The four molecules produce some tyrosine that I've just described or shown on the screen here. 04:13 Now I want to describe a little bit about each one of them. 04:15 L-DOPA is, as we saw, a precursor to dopamine. 04:19 It is a molecule that readily crosses the blood-brain barrier. 04:22 And it's used to treat Parkinson's disease. 04:24 It's a very important treatment for Parkinson's disease. 04:29 Dopamine is a very important neurotransmitter. 04:32 It inhibits the release of norepinephrine. 04:35 And norepinephrine is a vasoconstrictor. 04:38 So by inhibiting the release of norepinephrine, dopamine is a vasodilator. 04:43 Dopamine reduces insulin production in the pancreas. 04:46 That's a very important function that has nothing to do with the brain. 04:50 Now deficiency of dopamine is what causes Parkinson's disease. 04:54 And that's why L-DOPA is used to treat Parkinson's disease. 04:57 Because by getting enough L-dopa into the brain, one hopes that the brain can synthesize enough dopamine to reduce the effects of the deficiency. 05:07 Dopamine has many, many neurological links to other problems and pathways. 05:11 And some of these links includes schizophrenia and ADHD. 05:16 Norepinephrine is a very important hormone. 05:19 It also a neurotransmitter and it works through the noradrenergic receptors. 05:25 Norepinephrine is part of what we refer to as the fight or flight response, which I've discussed in other lectures in this series. 05:32 It works by increasing the heart rate and blood pressure and that's why it is a vassal constrictor. 05:38 Epinephrine, also known as adrenalin is a related compound to norepinephrine. 05:42 It also is a hormone. 05:44 And it also has actions very similar to that of norepinephrine. 05:47 It's involved in the fight or flight response. 05:50 And like norepinephrine, it increases the heart rate and blood pressure.
About the Lecture
The lecture Aromatic Family: Phenylalanine and Tyrosine by Kevin Ahern, PhD is from the course Amino Acid Metabolism. It contains the following chapters:
- Phenylalanine
- Tyrosine
Included Quiz Questions
Which of the following is true regarding phenylalanine?
- Phenylalanine can cause brain damage in high amounts.
- Phenylalanine is a nonessential amino acid.
- Phenylalanine is made from tyrosine.
- Excess phenylalanine hydroxylase causes phenylketonuria.
Which of the following is true regarding tyrosine? Select all that apply.
- Tyrosine is essential only if phenylalanine is absent.
- Tyrosine is an aliphatic amino acid, i.e., it has no cyclic structures with alternating double-bond characteristics.
- Tyrosine is important in chlorophyll.
- A deficiency in 1 of the molecules derived from tyrosine causes Parkinson disease.
- Tyrosine is an important precursor of some neurotransmitters.
The deficiency of which enzyme leads to phenylketonuria?
- Phenylalanine hydroxylase
- Phenylalanine synthase
- Phenylalaninase
- Phenylalanine transaminase
- Phenylalanine kinase
Which of the following does not fall under the category of catecholamines?
- S-adenosylmethionine
- L-dopa
- L-dopamine
- Norepinephrine
- Epinephrine
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
The best explanation, Concise and to the point. Thank you so much.