Playlist
Show Playlist
Hide Playlist
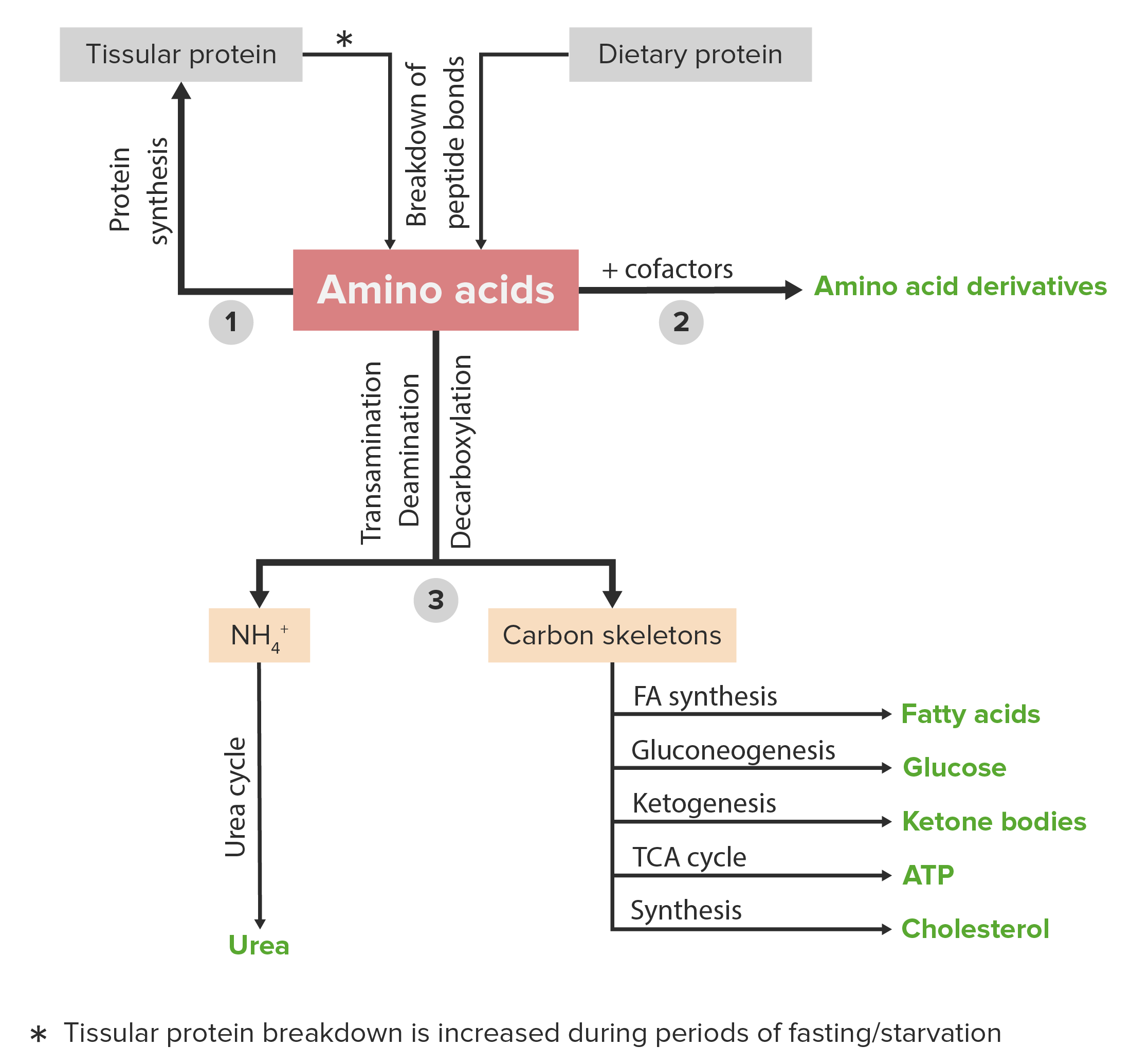
Amino Acid Catabolism
-
Slides AminoAcidMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
00:02 Wenn wir nun den Aminosäure-Katabolismus betrachten, habe ich bereits über einige der einzelnen Reaktionen gesprochen, möchte ich den Abbau jetzt in seiner Gesamtheit aller Aminosäuren beschreiben. 00:12 Der Aminosäureabbau wird in drei Kategorien unterteilt. 00:15 Die erste Kategorie sind Aminosäuren, die glucogen sind. 00:18 Dies sind Aminosäuren, die aufgespalten werden und als Zwischenprodukte in der Glykolyse oder Glukoneogenese dienen. 00:24 Und die glucogenen Aminosäuren sind in der der Abbildung rechts grün markiert. 00:29 Die ketogenen Aminosäuren sind diejenigen, die zu Acetyl-CoA abgebaut werden. 00:34 Und sie sind in der Abbildung auf der rechten Seite in rot dargestellt. 00:37 Es gibt einige Aminosäuren, die als Zwischenstufen in beiden Wegen auftreten und so auf dem ketogenen und dem glukoneogenen Weg vorkommen können. 00:43 Und diese Aminosäuren sind im Pfad in blau dargestellt. 00:49 Die Wege, die auf der rechten und linken Seite schematisch dargestellt sind, beginnen mit auf dem Glykolyse-Weg mit Glukose und enden mit Pyruvat. 00:58 Und der Kreis auf der rechten Seite stellt den Zitronensäurezyklus und die Zwischenprodukte des des Zitronensäurezyklus dar. 01:05 Wir können also sehen, wie der Abbau der Aminosäuren in diesen einzelnen Pfaden funktioniert. 01:12 Hier sind die glucogenen Aminosäuren, als Beispiel sehen Sie hier Alanin, da ich nicht alle beschreiben werde. 01:16 Sie können außerdem die Liste der vorhandenen Aminosäuren sehen. 01:19 Die ketogenen Aminosäuren sind nicht annähernd so stark gebunden, aber es gibt nur zwei, die in erster Linie das Acetyl-CoA produzieren, Lysin und Leucin. 01:27 Und hier sind die Aminosäuren, die an beiden beteiligt sind. 01:30 Ja, es ist kompliziert. Es sieht kompliziert aus. 01:32 Aber es gibt tatsächlich eine Vereinfachung. 01:35 Und das ist, dass es nur sechs Moleküle sind, die am Katabolismus von Aminosäuren beteiligt sind. 01:41 Sie werden hier gezeigt. 01:42 Pyruvat, Acetyl-CoA, Oxalacetat, Alpha-Ketoglutarat, Succinyl-CoA und Fumarat. 01:49 Alle Animosäuren können aufgespalten werden, um diese sechs Zwischenprodukte zu erzeugen. 01:56 Sie können sehen, dass vier von diesen einzelnen Moleküle hier oben in den Zitronensäure- Zyklus eingehen, deshalb ist klar, dass die Beschreibung des Zitronensäurezyklus obligatorisch ist. 02:06 Er kann auch Zwischenprodukte von anderswo abbauen. 02:09 Er kann auch eine Quelle für Zwischenprodukte sowie für die Herstellung einiger dieser Aminosäuren sein. 02:14 Der Aminosäurekatabolismus ist also ziemlich wichtig, wie wir sehen können. 02:18 Sie müssen in der Lage sein, mit diesen Mitteln umgehen zu können. 02:20 Die Zelle muss in der Lage sein, die richtige Menge an Aminosäuren herzustellen und nicht zu viel Ammoniak zu produzieren, wenn sie optimal funktionieren will. 02:29 Die meisten dieser Wege führen letztlich zu Aminen durch Transaminierung. 02:34 Aber auch die zusätzliche Bewegung von Ammoniak ist ebenfalls ein wichtiger Aspekt. 02:39 Deshalb ist die Transaminierung von Glutamat wichtig, weil sie Amine übertragen oder aufnehmen kann und so kann auch Ammoniak verarbeitet werden. 02:48 Glutamat ist also wichtig für den Transport all dieser Stickstoffverbindungen. 02:52 Die Verbindungen transportieren diese Stickstoffverbindungen weg von empfindlichem Gewebe und transportieren sie zur Leber. 02:56 Und wir haben gesehen, wie der Glukose-Alanin- Zyklus zum Beispiel dem Gehirn hilft, dass überschüssige Amin abzubauen ohne Glutamat zu verlieren. 03:05 In der Leber wird das Ammoniak für die Harnstoffsynthese und für die Ausscheidung verwendet. 03:10 Die Leber erledigt eine Menge Dinge in unserem Körper. 03:13 Das Gehirn ist, wie ich schon sagte, empfindlich gegenüber Ammoniakwerten und deshalb muss es sehr vorsichtig sein, um ein angemessenes Gleichgewicht zu finden. 03:20 Glutamat ist ein Neurotransmitter, wie ich schon sagte, und das ist warum der Glukose-Alanin- Stoffwechselweg wichtig ist. 03:25 Alanin bietet eine Möglichkeit, dass Amin zu erhalten und das Ammoniak aus dem Gehirn zu entfernen. 03:31 Wie Sie sich vielleicht vorstellen können, haben wir hier einige komplizierte Wege. 03:34 Ich habe über einige der Krankheiten gesprochen, die damit verbunden sind. 03:37 Ich möchte einige von ihnen überprüfen und einige der folgenden Punkte ansprechen, die am Aminosäureabbau beteiligt sind. 03:43 Alcaptonurie ist, wie wir gesehen haben, beteiligt am Katabolismus von Phenylalanin und Tyrosin beteiligt. 03:49 Mathylmalonsäureanämie, die ich nicht beschrieben habe, entsteht durch Defekte im Katabolismus von Methionin, Threonin, Isoleucin und Valin. 03:57 Die Mapel-Sirup-Krankheit ist entstanden aus den Problemen des Zusammenbruchs in Verbindung mit den verzweigtkettigen Aminosäuren. 04:04 Homocystinurie entsteht durch Probleme beim Abbau von Methionin. 04:10 Tryrosinämie, natürlich, aus den Abbauprodukten von Tyrosin. 04:14 Und Argininämie entsteht durch Probleme im Zusammenhang mit dem Abbau von Arginin. 04:18 Und darüber werde ich mehr sagen in der Vorlesung über den Harnstoffzyklus. 04:21 Hyper-Methioninämie tritt auf, und ihr Name deutet darauf hin, bei Mängeln beim Abbau von Methionin. 04:27 Hyperlysinämie durch Mangelzustände beim Abbau von Lysin. 04:31 Glycin-Enzephalopathie durch den Defizite beim Abbau von Glycin. 04:35 Propionsäureanämie durch Mangelerscheinungen zum Abbau dieser vier Enzyme. 04:39 Und schließlich die Hyperprolinämie durch Defizite beim Abbau von Prolin. 04:44 Mit den Aminosäuren passiert nun unter anderem folgendes, was wir auch chemisch betrachten müssen, nachdem sie in Proteine eingebaut worden sind, werden viele von ihnen chemisch verändert. 04:53 Diese chemische Modifikation verbessert tatsächlich die Fähigkeit eines Proteins zu funktionieren oder kann wichtige Dinge in Bezug auf die Signalisierung, die an Proteinen oder Protein-Protein-Wechselwirkungen beteiligt sind, bewirken. 05:05 Die am häufigsten chemisch modifizierten Aminosäuren sind hier auf dem Bildschirm zu sehen. 05:10 Ich habe zwei Beispiele in Bezug auf die Struktur. 05:12 Und Sie können sich vorstellen mit Ihrem Wissen über Chemie, wie die anderen tatsächlich aussehen. 05:18 Hydroxylysin ist eines. 05:19 Es ist sehr häufig modifiziert, wie wir gesehen haben. 05:22 Und Phosphotryrosin ist wichtig für den Signalisierungsprozess. 05:25 Serin ist in der Zelle ein Ziel für Glykosylierung und Phosphorylierung. 05:30 Dies ergibt sich aus der Seitenkette von Serin, die eine Hydroxylgruppe enthält, und das ist der Ort, an dem ein Phosphat durch eine Kinase oder ein Zucker im dem Prozess der Glykosylierung angehängt werden kann. 05:41 Threonin hat die gleiche Seitenkette wie das Serin, eine Hydroxylgruppe. 05:45 Und wie Serin ist es ein Ziel für Phosphorylierung und Glykosylierung. 05:50 Lysin ist eine wirklich komplizierte Aminosäure, was die Modifikation angeht. 05:56 Die häufigsten Modifikationen sind Methylierung und Acetylierung und Hydroxylierung. 06:01 Nachfolgend habe ich eine Reihe anderer Arten von Modifikationen aufgelistet, die mit Serin geschehen können. 06:06 Ich werde sie hier nicht alle aufzählen. 06:07 Aber es genügt zu sagen, dass Lysin, dass am meisten verbreitete kovalent modifiziertes Enzym, das in Proteinen vorkommt, ist. 06:14 Methionin wird in Prokaryoten durch das Hinzufügen einer formalen Gruppe modifiziert, wie ich es bereits in den Vorlesungen über den Aminosäurestoffwechsel beschrieben habe. 06:22 Und das geschieht nur bei Prokaryoten, nur bei den Aminosäuren, die in Proteinen von Prokaryoten vorkommen. 06:28 Arginin kann acetyliert werden und die Acetylierung von Aminosäuren wie Arginin und auch Lysin sind wichtig, weil sie positive Ladungen haben. 06:37 Und die Hinzufügung einer Acetylgruppe verändert diese positive Ladung in eine neutrale Ladung. 06:42 Wenn wir über Histone sprechen, wo diese Modifikation stattfindet, müssen wir feststellen, dass Histone sehr positiv geladene Proteine sind, die mit der DNA, die negativ geladen ist, interagieren. 06:53 Wenn wir also folgendes nehmen: Wir haben Arginin oder Lysin und wandeln es von einer positiven Ladung in eine Nullladung um, verändern wir die Anziehungskraft, die zwischen zwischen dem Histon und der DNA herrscht. 07:05 Und wir werden mehr darüber in unserer Diskussion über die Kontrolle der Genexpression der DNA sprechen. 07:13 Prolin ist ein Ziel für die Hydroxylierung. 07:15 Das passiert natürlich auch in Kollagen, wie ich in der Kollagenvorlesung beschrieben habe. 07:19 Cystein ist, wie ich bereits erwähnt habe, eine sehr wichtige Aminosäure, denn innerhalb von Proteinen kann es mit einem zweiten Cystein reagieren kann. 07:26 Zwei Cysteine können also zusammenkommen und eine Disulfidbindung eingehen. 07:30 Ich habe dies bereits in den Vorlesungen über den Aminosäurestoffwechsel erwähnt. 07:34 Und diese Disulfidbindung, die sich zwischen zwei Cysteinen bildet, ist eine sehr wichtiges Strukturmerkmal, um Proteine zu stabilisieren. 07:41 Histidin ist nicht sehr stark modifiziert. 07:44 Gelegentlich wird aber auch Histidin phosphoryliert. 07:47 Das ist etwas, das wir erst in den letzten Jahren herausgefunden haben. 07:50 Die Bedeutung dieser Phosphorylierung ist derzeit noch nicht ganz klar. 07:54 Glycin ist ein Ziel für die Myristoylierung. 07:57 Dies beinhaltet die Bindung von einer Myristinsäure an ein Glycin. 08:02 Glutaminsäure ist ein Ziel für die Carboxylierung, und darüber habe ich bereits in Bezug auf den Prozess der Blutgerinnung gesprochen. 08:12 Und schließlich ist Asparagin ein Ziel für Glykosylierung, genau wie das Serin und Threonin, was bedeutet, dass eine Seitenkette der Aminosäure mit Zuckerresten verbunden wird. 08:24 In dieser Reihe von Vorlesungen habe ich die beteiligten Stoffwechselwege der 20 Aminosäuren, plus der einen seltene Aminosäure, Selenocystein, beschrieben. 08:34 Hier gibt es eine große Vielfalt. 08:35 Es gibt eine Menge verschiedener Reaktionen, die in einer Vielzahl von verschiedenen Kontrollsystemen des Körpers eingebaut sind. 08:39 Dies ist notwendig, wie wir beschrieben haben, um die einzelnen Aminosäuren auszugleichen. 08:44 Wir haben auch gesehen, wie diese Aminosäuren chemisch modifiziert werden und wie der Abbau dieser Aminosäuren zu zahlreichen genetischen Krankheiten führen kann.
About the Lecture
The lecture Amino Acid Catabolism by Kevin Ahern, PhD is from the course Amino Acid Metabolism. It contains the following chapters:
- Amino Acid Catabolism
- Amino Acids Modified After Incorporation Into a Protein
Included Quiz Questions
Which of the following is true regarding amino acid catabolism?
- Glucogenic amino acids include those broken down into pyruvate and oxaloacetate.
- Ketogenic amino acids break down to ketone bodies, such as acetone.
- Most amino acids are ketogenic.
- Ketogenic amino acids are broken down into gluconeogenesis intermediates.
- Acetyl-CoA is a common intermediate for glucogenic amino acids.
Which of the following is true regarding post-translational modification of amino acid side chains?
- It involves phosphorylation of serine and threonine.
- It involves carboxylation of arginine.
- It results in glycosylation of proline.
- It results in the decarboxylation of tyrosine.
- It results in phosphorylation of glycine.
Which of the following are ketogenic amino acids?
- Lysine and leucine
- Isoleucine and valine
- Tyrosine and glutamate
- Glycine and asparagine
- Threonine and serine
Which of the following is not correctly associated regarding the defective amino acid catabolism?
- Alcaptonuria — alanine and threonine
- Methylmalonic acidemia — methionine, threonine, isoleucine, and valine
- Homocystinuria — methionine
- Glycine encephalopathy — glycine
- Propionic acidemia — methionine, threonine, isoleucine, and valine
Which of the following statements is NOT true regarding amino acid modifications?
- The disulfide bond formations between the two cysteine residues of a protein molecule give rise to an unstable structure.
- The myristoylation of glycine involves the addition of myristic acid to the glycine.
- Phosphotyrosine plays an important role in the cell signaling process.
- Lysine amino acid commonly undergoes methylation, acetylation, and hydroxylation modification.
- Asparagine may be modified by phosphorylation.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Explains everything extremely clearly and made all of the processes easy to understand. This was a great lecture series!