Playlist
Show Playlist
Hide Playlist
α-ketoglutarate Family and Glutamine Synthesis Uses Glutamine Synthetase
-
Slides AminoAcidMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
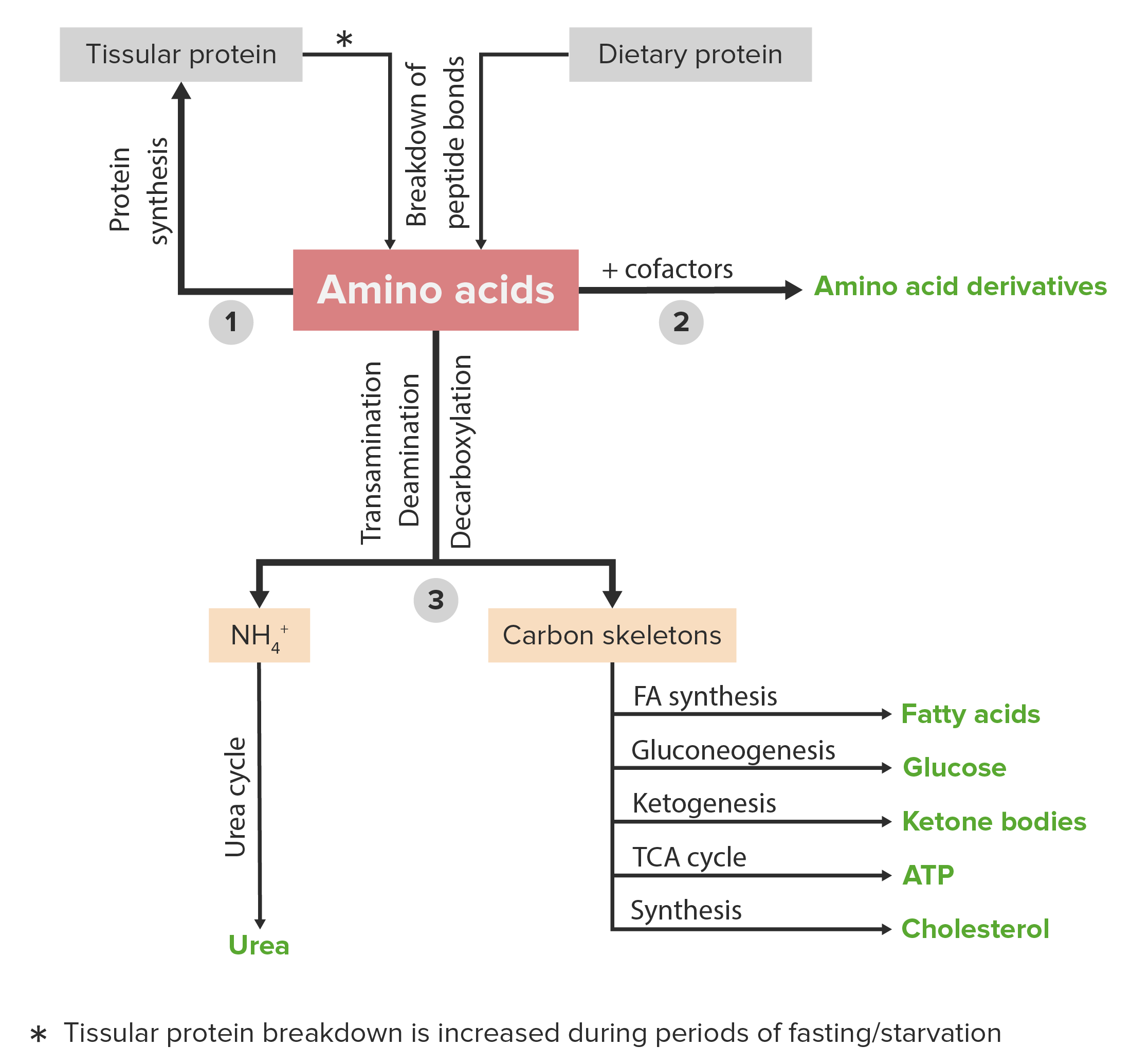
00:02 I want to start a discussion here talking about the alpha-ketoglutarate family, also called the glutamate family because all of the amino acids that are made in this family include glutamate and alpha-ketoglutarate as precursors. 00:14 Now, this is a very good place to start our discussion because the alpha-ketoglutarate family starts with the process that's very central to many amino acid metabolic processes. 00:24 And this pathway is known as transamination. 00:28 So, transamination occurs when an alpha-keto acid is converted into an amino acid. 00:35 And that requires a donor of amine group which is in this case described as amino acid X, and that amino acid X after donating its amine group becomes an alpha-keto acid. 00:46 So, we sort of see this swapping. 00:47 Well, that's a lot of words. 00:49 Let's take a look at what actually happens in the process. 00:52 So, we see here alpha-ketoglutarate, our precursor of all the amino acids in this family that I've described. 00:58 We see to its right the amino acid aspartate. 01:01 Aspartate is the donor of the amine group that will help to make glutamate. 01:07 We see in this process that's starting at the top on the left, alpha-ketoglutarate becomes glutamate on the bottom left and the process aspartate or aspartic acid becomes oxaloacetate on the lower right. 01:21 So, this is now showing us structurally what's happening with the words that I've showed in the previous slide. 01:27 The alpha-keto acid number one is alpha-ketoglutarate. 01:31 The amino acid number two is glutamate. 01:34 The amino acid number one, our starting amino acid or amino acid X, is aspartate. 01:39 And our final alpha-keto acid X is oxaloacetate. 01:44 We can actually see very simply what's happening right here. 01:47 We see the oxygen on alpha-ketoglutarate. 01:50 And we see the amine on aspartate. 01:52 They're basically swapping. 01:53 And here's that swap that they do. 01:55 The oxygen goes from alpha-ketoglutarate to oxaloacetate and goes from aspartate to make glutamate. 02:02 This is central to transamination and every transamination that I've described will have exactly this going on. 02:09 The molecules and other transaminations will differ in terms of what the starting materials are but all that's happening are the swapping I've described here. 02:17 So having understood now of what the process of transamination is, we can begin to understand metabolism that's happening among many amino acids. 02:26 The next amino acid that I want to describe the synthesis of is glutamine. 02:30 And glutamine turns out to be central to amino acid metabolism for a very different reason. 02:35 And it actually relates to another lecture I will give in this series relating to the urea cycle. 02:40 And we'll see what's happen here. 02:42 Glutamine is really important for what I've describe as nitrogen metabolism And when we talk about nitrogen metabolism, we're really talking about something that all amino acids have to have. 02:52 The amine of course containing a nitrogen. 02:54 Well, not all nitrogens get on to amino acids as a result of transamination. 02:59 It's a very common way, but not the only way. 03:02 In making glutamine, glutamine is made on a very simple reaction from glutamate as we see here. 03:07 And we've already seen how glutamine is made. 03:10 The enzyme that catalyzes this reaction is known as glutamine synthetase. 03:13 As you can see it requires ATP. 03:16 But we see that the nitrogen source here is ammonia or in this case ammonium ion which is the same in aqueous solution. 03:23 This ammonium ion is produced as a byproduct of breakdown of other amino acids. 03:30 And that's pretty straight forward except when we consider that ammonium ion or ammonia is toxic. 03:36 So, people talk about detoxing and all that sort of things when they talk about their bodies and their health and so forth. 03:41 And they, a lot of the cases, don't understand what that actually means. 03:44 But we talk about detoxifying something. 03:46 This is a reaction that actually detoxifies ammonia because it's grabbing free ammonium which is toxic and putting it on to amino acid to make glutamine. 03:56 Now, this enzyme that catalyzes this reaction is a very complicated enzyme. 04:00 We're not going to go into all the details of it but suffice it to say that this reaction is important to control. 04:07 Cells don’t' want to make too much of anything and so this reaction is important for grabbing ammonium but if the cells makes too much glutamine, it will have other problems. 04:16 So, therefore it's important to control how much glutamine is being made. 04:20 This enzyme is regulated by a wide variety of things and some of them are shown on the screen here. 04:25 These are things that inhibit this enzyme. 04:28 The molecules that I have drawn the boxes around are all molecules that are made from glutamine. 04:35 We see histidine and tryptophan among the amino acids. 04:38 We see AMP and CTP among the nucleotides. 04:41 And we see carbamoylphosphate and glucosamine-6-phosphate among other molecules. 04:46 All of these are made from glutamine. 04:48 So, why is it important that these things inhibit the glutamine synthetase? The reason it's important is because as they start to accumulate, it means that the cell has abundant glutamine. 04:59 If they get too high, glutamine's too high. 05:01 If they get too high, they turn off this enzyme. 05:03 So, there's a balance that's actually happening in controlling the synthesis of glutamine. 05:10 Now, the alpha-ketoglutarate family is important because, as I said, we have to control how many different things are made particularly of glutamine. 05:17 So glutamine is very central to metabolism of all the amino acids that's happening. 05:23 Glutamine synthetase has multiple sites and that's actually how it controls the synthesis of glutamine. 05:31 Those multiple sites are not for making glutamine but rather for binding inhibitors. 05:35 So, you saw on the last slide about 10 different inhibitors that can affect the enzyme. 05:40 And there are multiple sites on the glutamine synthetase; one for each of the various inhibitors. 05:45 So, as we look at the amount of inhibition that the enzyme exhibits, it's actually a function of how many of those sites are bound to inhibitors. 05:55 The more inhibitors are present, the more sites will be bound and the more the enzyme will be inhibited. 06:01 It's important that the enzyme not have a simple on/off control as we had seen other enzymes. 06:08 But rather that we have an up-down regulation where we make it more active or less active depending upon how much these are the molecules are needed. 06:17 The glutamine synthatase is important, as I've said, for removing ammonia. 06:21 And this is important in many places in the body but particularly in the brain. 06:25 As we'll see in some of the other lectures in these series, the accumulation of ammonia in the brain has very important problems neurologically. 06:36 Ammonia is produced by reducing nitrites which can be found in the diet, by amino acid metabolism and also by photorespiration which occurs in plants. 06:46 In the liver, glutamine oxidation, by glutamate dehydrogenase actually releases ammonia for release -- I'm sorry, for synthesis of urea. 06:54 And urea is the excretion product by which we get rid of excess amines that appear in our body. 07:03 In addition to allosteric regulation of glutamine synthetase that I've just described, glutamine synthetase can also be regulated by covalent modification. 07:10 And we can see that occurring on this slide right here. 07:14 Looking at the enzyme in the unadenylated form, which is the form before the modification occurs, we see that it is the most active or the more active form of the enzyme. 07:23 The addition of an adenyl group comes from ATP and the reaction that you can see here, catalyzed by adenylyl transferase. 07:30 This reaction is facilitated by an additional protein known as P sub A. 07:36 The unadenylated form of glutamine synthetase that is the covalently modified form is less active. 07:41 So we see this addition of this adenylyl group actually helps the enzyme -- helps to control the enzyme in a different way besides allosterically. 07:49 The adenylyl tranferase can in fact remove the adenyl group as I've described here using a different protein called P sub D. 07:59 And this reaction is a phosphorolysis which actually uses a phosphate to remove the adenyl group from the adenylyl glutamine synthetase.
About the Lecture
The lecture α-ketoglutarate Family and Glutamine Synthesis Uses Glutamine Synthetase by Kevin Ahern, PhD is from the course Amino Acid Metabolism. It contains the following chapters:
- Glutamate
- Glutamine Synthesis Uses Glutamine Synthetase
- Alpha-ketoglutarate Family
Included Quiz Questions
Which of the following statements is correct regarding glutamate?
- In transamination, glutamate is produced when alpha-ketoglutarate accepts an amine group from another molecule.
- Synthesis occurs when glutamine gains an amine group.
- Glutamate is an important precursor of aspartate.
- Glutamate is produced by the oxidation of arginine.
What molecular change occurs during transamination?
- An amine group and a carbonyl group are exchanged.
- Decarboxylation
- Carboxylation accompanies addition of an amine group.
- Carbons are exchanged between 2 molecules.
- The amine group on 1 molecule is exchanged with an amine group on another molecule.
The conversion of glutamate to glutamine catalyzed by glutamine synthetase results in which of the following?
- Removal of ammonia
- Production of a toxic product
- Production of ATP
- Phosphorylation of ADP
- Release of urea
Which of the following reactions is NOT responsible for producing cellular ammonia?
- Decarboxylation
- Nitrite reduction
- Glutamate oxidation
- Amino acid metabolism
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
I cannot believe I am just now finding this resource during my dedicated Step 1 period. However, I am so glad that I found it now, right at the beginning of Step prep, rather than later. I plan to use this as one of my main resources for the remainder of time up until my exam. I realized while reading First Aid that, although I had known the subjects very well at one point, that my foundation was not as solid as it once was. This is perfect for reviewing and providing the big picture for everything. It's also an amazing resource for providing the "why" and "how" behind all the small factoids written in First Aid which is just what I need. It's so much easier to retain the information when you actually understand what is going on rather than memorizing a 650+ page book of bullet point lists/facts. This is great. I was a huge fan of Boards and Beyond, but I have since ditched that resource and am fully focusing on this one since this seems a lot more comprehensive and better taught (though Dr. Ryan has also done an amazing job too). Thank you guys so much for coming out with such a well thought out and developed and thorough resource. This is going to make board prep so much smoother. If I end up scoring highly on Step 1, I will make sure to write a review on Reddit, SDN, etc raving about Lecturio.