Playlist
Show Playlist
Hide Playlist
Allosteric Control – Metabolic Control of Enzyme Activity
-
10 Advanced MetabolicControlOfEnzymeActivity.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
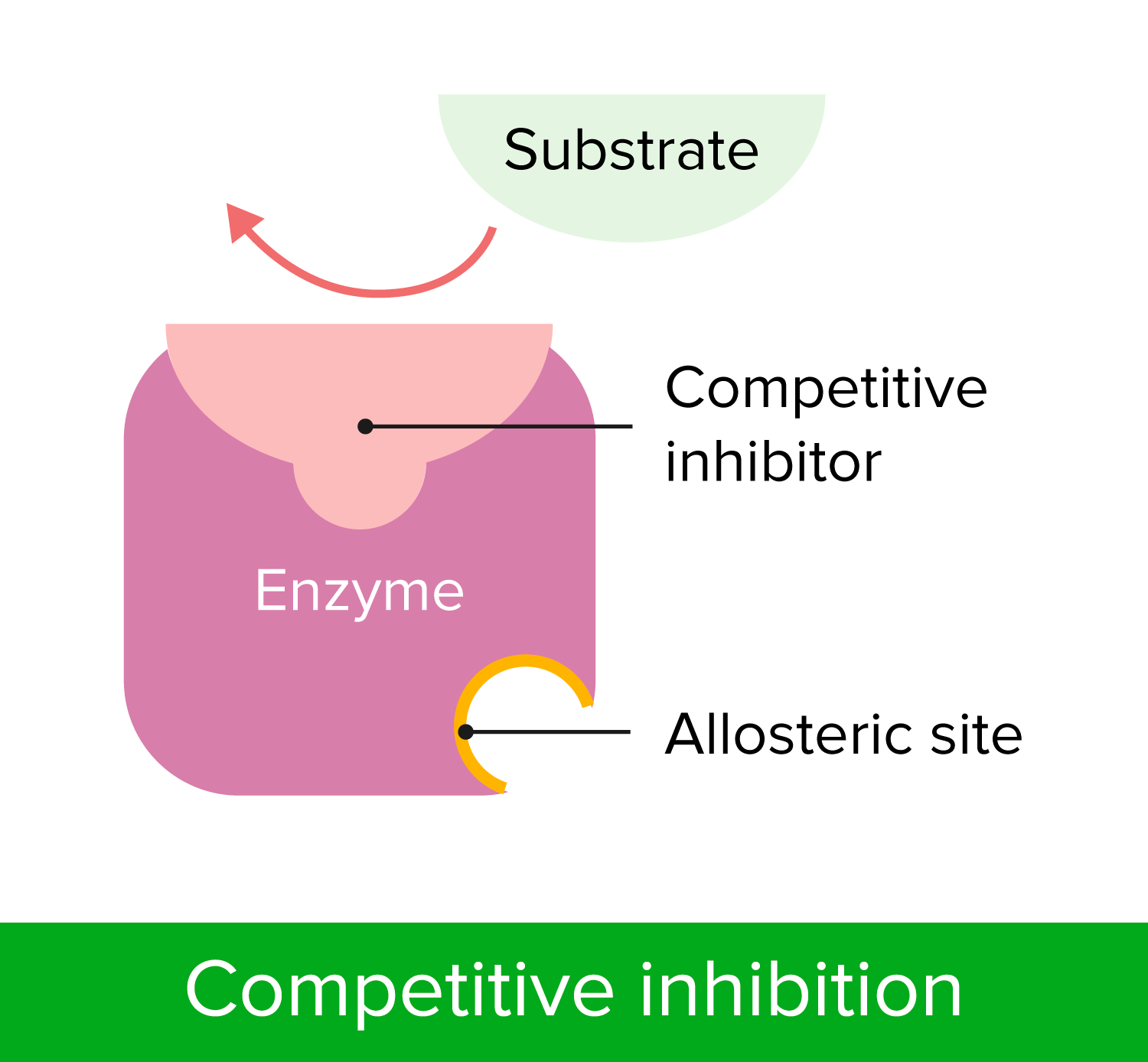
00:01 Der erste Mechanismus, über den ich sprechen möchte ist die allosterische Bindung. 00:04 Ich habe dies bereits in einer anderen Präsentation besprochen. Ich werde es also ziemlich schnell durchgehen. 00:07 Wir erinnern uns an die Diskussion über Enzyme. Enzyme, die der Michaelis-Menten-Kinetik gehorchen zeigen ein kinetisches Profil, wie wir es hier auf dem Bildschirm sehen. Eine hyperbolische Darstellung und die Darstellung von V gegen S. V ist die Geschwindigkeit der enzymatischen Reaktion. 00:21 Jetzt haben wir auch erwähnt, dass Enzyme, die sich nicht nach der Michaelis-Menten-Kinetik verhalten oft in einer sigmoidalen Darstellung gezeigt werden. 00:30 Nun spreche ich in diesem Vortrag über die Gründe dafür. 00:35 Im ersten Szenario verändert das Substrat das Enzym nicht. 00:38 Das heißt, wenn das Enzym das Substrat bindet, ist alles, was passiert, nur, dass das Enzym die Reaktion mit dem Substrat katalysiert. 00:45 Im zweiten Szenario jedoch wird das Substrat durch die Wechselwirkung mit dem Enzym verändert und verändert die Art und Weise, wie das Enzym an das Substrat bindet. 00:55 Wenn es sich bei dem Enzym also um ein Enzym mit mehreren Untereinheiten handelt, wie es viele Enzyme sind, dann kann die Bindung des ersten Substrats die Bindung der nachfolgenden Substrate beeinflussen. 01:03 Und deshalb weicht diese Gruppe von der hyperbolischen Kurve ab, die wir bei der ersten Gruppe gesehen haben. 01:09 Nun, das klassische Enzym zur Untersuchung der allosterischen Bindung ist die Aspartat-Transcarbamoylase, die Sie auf dem Bildschirm sehen können. 01:16 Die Aspartat-Transcarbamoylase ist auch bekannt als ATCase, weil es sich leichter aussprechen lässt. 01:23 Die Aspartat-Transcarbamoylase hat eine Struktur wie die, die Sie auf dem Bildschirm sehen. 01:26 Sie enthält 12 Proteine, davon 6 Untereinheiten, die wir katalytisch nennen und 6, die wir als regulatorisch bezeichnen, wie wir sehen werden. 01:36 Nun katalysiert die ATCase eine Reaktion, die für die Synthese von Pyrimidinen notwendig ist. 01:42 Wir können die Reaktion auf dem Bildschirm sehen. Diese Reaktion ist wichtig für die Zellen. 01:45 Die eigentliche Reaktion ist für uns nicht so wichtig, außer für eines der Substrate. 01:50 Aspartat ist eine Aminosäure und eines der Substrate der Reaktion. 01:57 Das ist diejenige, die uns hier interessiert. 01:59 ATP ist eine hohe Energiequelle und es ist, wie wir bereits besprochen haben, das Benzin der Zelle, das die gesamte Energie liefert, die die Zelle braucht. 02:10 Darüber hinaus liefert ATP nicht nur eine hohe Menge an Energie, sondern wird auch für die Herstellung von RNA und DNA benötigt, und wenn wir darüber nachdenken so ist es auch das Endprodukt des des Weges, der durch die ATCase initiiert wird. 02:23 Die ATCase leitet einen Weg zur Synthese von CTP ein. Das Endprodukt dieses Weges ist CTP. 02:33 CTP wird auch zur Herstellung von RNA und DNA verwendet. 02:38 Hier sind die Substrate der Reaktion und hier ist das Enzym, das diese Reaktion katalysiert. 02:44 Wenn Aspartat an die ATCase bindet, verändert es das Enzym und es verändert spezifisch die Affinität des Enzyms für zusätzliche Aspartate. 02:52 Deshalb sehen wir den sigmoidalen Verlauf des Diagramms. 02:54 Die ATCase wird also durch das Aspartat beeinflusst. 02:58 Es ist wichtig zu wissen, dass Enzyme in zwei verschiedenen Zuständen existieren können. 03:03 Der eine Zustand des Enzyms wird als R-Form bezeichnet und wir können uns das wie eine entspannte Form des Enzyms vorstellen. Und diese entspannte Form des Enzyms begünstigt die Bindung von zusätzlichen Substraten. 03:16 Der andere Zustand des Enzyms wird als T-Form oder der enge Zustand bezeichnet und ist der gespannte Zustand des Enzyms. 03:20 Hier wird zwar das Substrat gebunden, aber nicht annähernd so gut wie im R-Zustand. 03:26 Der R-Zustand ist also die aktivere Form des Enzyms und der T-Zustand ist die weniger aktive Form des Enzyms. 03:34 Beachten Sie, dass beide Formen aktiv sind. Es handelt sich also nicht um einen Ein-Aus-Schalter, sondern vielmehr verändert sich die Menge der Aktivität des Enzyms. 03:42 Dieses Diagramm veranschaulicht wie sich diese sigmoidale Kurve nicht durch die Bindung von Aspartat, sondern durch die Bindung von ATP verändert. 03:53 Es hat sich herausgestellt, dass ATP auch dieses Enzym beeinflusst. 03:58 ATP ist kein Substrat des Enzyms. 03:59 Und ATP ist, wie bereits erwähnt, eine Energiequelle und es tritt auch in Erscheinung. 04:06 Eine Energiequelle ist wichtig, denn, Zellen, die sich replizieren müssen ihre DNA kopieren und dazu müssen sie mehr Nukleotide herstellen. 04:17 Wenn sich die Zelle in einem hochenergetischen Zustand befindet, ist sie in einer besseren Position, das zu tun, als in einem Zustand mit niedriger Energie. Wenn sie sich in einem Zustand niedriger Energie befindet würde die Zelle ein großes Risiko bei der Herstellung von Nukleotiden eingehen. 04:31 Wenn sich nun die Zelle im Hochenergiezustand befindet, ist ATP im Überfluss vorhanden. 04:35 Wenn das passiert, bindet es an die ATCase und bewirkt, dass sich die Kurve, wie Sie hier sehen, verschiebt. 04:41 Sie verschiebt sich von der blauen Linie, mit der wir begonnen haben zur orangefarbenen Linie, die hier gezeigt wird. 04:47 Der Unterschied zwischen diesen beiden ist, dass bei der orangefarbenen Linie, die nach links verschoben ist, die Reaktion schneller katalysiert wird bei einer niedrigeren Konzentration des Substrats. 04:56 Die gleiche Konzentration an Substrat führt also zu einer erhöhten Geschwindigkeit des Enzyms. 05:02 ATP hat Folgendes bewirkt: die Aktivierung des Enzyms. 05:08 Es sagt dem Enzym "Es ist okay, mach weiter und mach Nukleotide, denn wir sind für die Replikation bereit." Nun aktiviert ATP die ATCase durch Überführung in den R-Zustand. 05:23 Ebenso überführt Aspartat das Enzym in den R-Zustand. 05:26 Das bedeutet also, dass das Enzym in der Lage ist, mehr Substrat effektiver zu binden und schneller zu arbeiten, weshalb wir einen Anstieg der Geschwindigkeit sehen. 05:38 Die Aktivität der ATCase wird durch eine dritte Verbindung beeinflusst, die hier auf dem Bildschirm zu sehen ist. 05:44 Die dritte Verbindung ist das Nukleotid CTP oder das Cytidintriphosphat. 05:50 Und das ist Cytidintriphosphat und sein Effekt, den Sie auf dem Bildschirm sehen. 05:55 Je mehr Cytidintriphosphat hinzugefügt wird, desto geringer ist die Geschwindigkeit der enzymatischen Reaktion, die von der ATCase katalysiert wird. 06:01 CTP ist also das Endprodukt des Weges, der durch die ATCase-Reaktion eingeleitet wird. 06:08 Wenn die Zelle zu viel CTP produziert, beginnt CTP an das Enzym zu binden und versetzt das Enzym in den T-Zustand. Wenn es sich in den T-Zustand versetzt, ist das Enzym weniger in der Lage an das Substrat zu binden. 06:25 Mit zunehmender Menge an CTP wird dies möglich. Dies erweist sich als wichtig, denn die Zelle will nicht zu viel CTP produzieren, denn wenn sie zu viel CTP produziert, ist das Energieverschwendung und das ist einer der Gründe. Ein weiterer Grund ist, dass Zellen nicht zu viele Nukleotide herstellen wollen. 06:41 Wenn die Nukleotide aus dem Gleichgewicht geraten, ist die Zelle viel anfälliger für Mutationen. 06:46 Das Gleichgewicht der Nukleotide und die Kontrollen, die ich hier beschrieben habe, sind nicht nur für Energiezwecke wichtig, sondern auch für die Aufrechterhaltung der Integrität der DNA. 06:57 Sie können sehen, dass die Beziehung zwischen der Geschwindigkeit und der Konzentration von CTP nicht genau linear ist. 07:04 Der wichtigste Punkt ist jedoch, dass sie abnimmt, wenn die Konzentration von CTP steigt. 07:11 Nun, Aspartat war ein Substrat, aber weder ATP noch CTP waren Substrate. Sie alle beeinflussten aber das Enzym. 07:19 Aspartat und ATP versetzen das Enzym in den R-Zustand und CTP verwandelt es in den T-Zustand. 07:27 Nun, wenn wir uns das Enzym ansehen, können wir große blaue Kugeln sehen, die katalytische Einheiten genannt werden. 07:33 Und das sind die Stellen, an denen die Reaktion katalysiert wird. 07:39 Die C-Einheiten, die katalytischen Einheiten, sind der Ort wo das Aspartat an das Enzym bindet. 07:47 Im Gegensatz dazu sind die grünen Kugeln, die mit Rs gekennzeichnet sind, regulatorische Untereinheiten. 07:51 Diese Kugeln sind die Stellen, an die entweder das ATP oder das CTP binden. 07:58 Und wenn das passiert, können wir sehen, dass das Enzym seinen Zustand umkehren kann, richtig? ATP bevorzugt also, wie gesagt, den R-Zustand. 08:06 CTP begünstigt den T-Zustand, und Aspartat begünstigt auch den R-Zustand. 08:12 Die Umkehrung in den R- oder T-Zustand kann also durch die Bindung eines dieser Proteine geschehen, die Sie auf dem Bildschirm sehen. 08:21 Um das Gesagte aufzufrischen und in der Grafik zu zeigen, möchte ich ein wenig darüber erzählen, was diese verschiedenen Teile der Kurve bedeuten. 08:29 Bei niedrigen Substratkonzentrationen, ist die ATCase-Aktivität gering und das bedeutet, dass sie sich im T-Zustand befindet. 08:38 Nun, wenn die Substratkonzentration für Aspartat steigt, beginnt das Enzym in den R-Zustand zu wechseln. Wir sehen also, dass dieser Wechsel geschieht und es in der Folge zur Veränderung der Steigung dieser Kurve kommt. 08:50 Und schließlich befindet sich die ATCase bei hohen Substratkonzentrationen meist im R-Zustand. 08:56 Das heißt, sie ist bereit, Nukleotide in Form von CTP zu produzieren. 09:02 Das erklärt also, dass die ATCase eine Struktur ist, deren Aktivität sich auf Aspartat bezieht und Sie wissen, wie sich das Enzym verändert in Bezug auf ATP und CTP. Aber es gibt noch eine weitere Sache zu berücksichtigen. 09:15 Das ist, dass ATP, wie ich bereits erwähnt habe, ein Purin ist und CTP ist ein Pyrimidin. 09:24 Wenn also die ATP-Konzentration hoch ist, bedeutet das, dass die Purinkonzentration hoch ist. 09:30 Und wenn wir uns die Nukleinsäuren ansehen, paaren sich Purine mit Pyrimidinen. 09:35 Zusätzlich zu einer hohen Energie zur Aktivierung dieses Enzyms haben wir ein Purin, das ein Enzym aktiviert, das Pyrimidine herstellt. 09:43 Dies ist eine sehr gute Möglichkeit, ein Gleichgewicht zwischen den Nukleotiden zu erreichen. Dies ist nötig aus den bereits erwähnten Gründen. 09:48 Die ATCase ist am wenigsten aktiv, wenn die Pyrimidin-Konzentration hoch ist. 09:52 Und wenn die Pyrimidin-Konzentration hoch ist, dann ist sie wahrscheinlich höher als bei den Purinen. 09:58 Dieses Gleichgewicht ist also wichtig und das Enzym hat all diese verschiedenen Überlegungen in seine Struktur eingebaut. 10:06 Eine weitere Besonderheit des ATCase-Systems, die ich den Studierenden ans Herz legen möchte, ist die Tatsache, dass es ein Paradebeispiel für ein Phänomen ist, das wir Rückkopplungshemmung nennen. 10:15 Die Rückkopplungshemmung tritt also auf, wenn das letzte Molekül in einem Stoffwechselweg sich anhäuft und dadurch das erste Enzym in diesem Stoffwechselweg gehemmt wird. 10:25 Diese Abbildung veranschaulicht dieses Prinzip deutlich. 10:28 Wir können sehen, dass die ATCase nicht die Bildung von CTP katalysiert Es katalysiert die Bildung von Dingen, die letztendlich zu CTP werden. 10:37 CTP, das Endprodukt dieses Prozesses hemmt das Enzym. 10:42 Das ist ein sehr effizienter Weg, um einen ganzen Stoffwechselweg zu kontrollieren, denn durch die Kontrolle der allerersten Reaktion und der Bildung des des allerersten Produkts, kontrolliert man den gesamten Weg. Denn wenn das erste Produkt nicht verfügbar ist, dann ist das zweite Produkt nicht verfügbar, usw. bis hin zu CTP. 11:02 Die Rückkopplungshemmung bietet also eine sehr effiziente Art und Weise, einen Signalweg zu kontrollieren.
About the Lecture
The lecture Allosteric Control – Metabolic Control of Enzyme Activity by Kevin Ahern, PhD is from the course Metabolic Control.
Included Quiz Questions
Which is true of allosteric control?
- It involves the binding of a small molecule to the enzyme.
- It acts to positively regulate enzymes, but not negatively.
- It involves the addition or removal of phosphates.
- It is basically an on/off switch.
Which is true of aspartate transcarbamoylase?
- it is activated by aspartate.
- It is allosterically regulated by its substrate, CTP.
- It is feedback inhibited by ATP.
- It is activated by CTP.
Aspartate transcarbamoylase does which of the following?
- Flips into the T state when CTP binds to the regulatory site.
- Flips into the R state when ATP binds to the catalytic site.
- Flips into the T state when aspartate binds to the regulatory site.
- Flips into the T state when CTP binds into the catalytic site.
Feedback inhibition occurs when which of these happens?
- An end product of a pathway inhibits the first enzyme of the pathway.
- A substrate inhibits an enzyme from catalyzing a reaction.
- A regulator binds to a catalytic site of an enzyme.
- The first product of a pathway inhibits the last enzyme of the pathway.
Which of the following do not control the activities of the ATCase enzyme?
- GTP
- TTP
- ATP
- CTP
- Aspartate
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
I liked the way the R and T states where explained. It all became very clear
A great, insightful lecturer. A Prof. at the top of his game. Miss his long hair though (checkout his youtube site)!