Playlist
Show Playlist
Hide Playlist
Acid Sensitivity – Pharmacokinetics and Pharmacodynamics
-
Slides 12 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
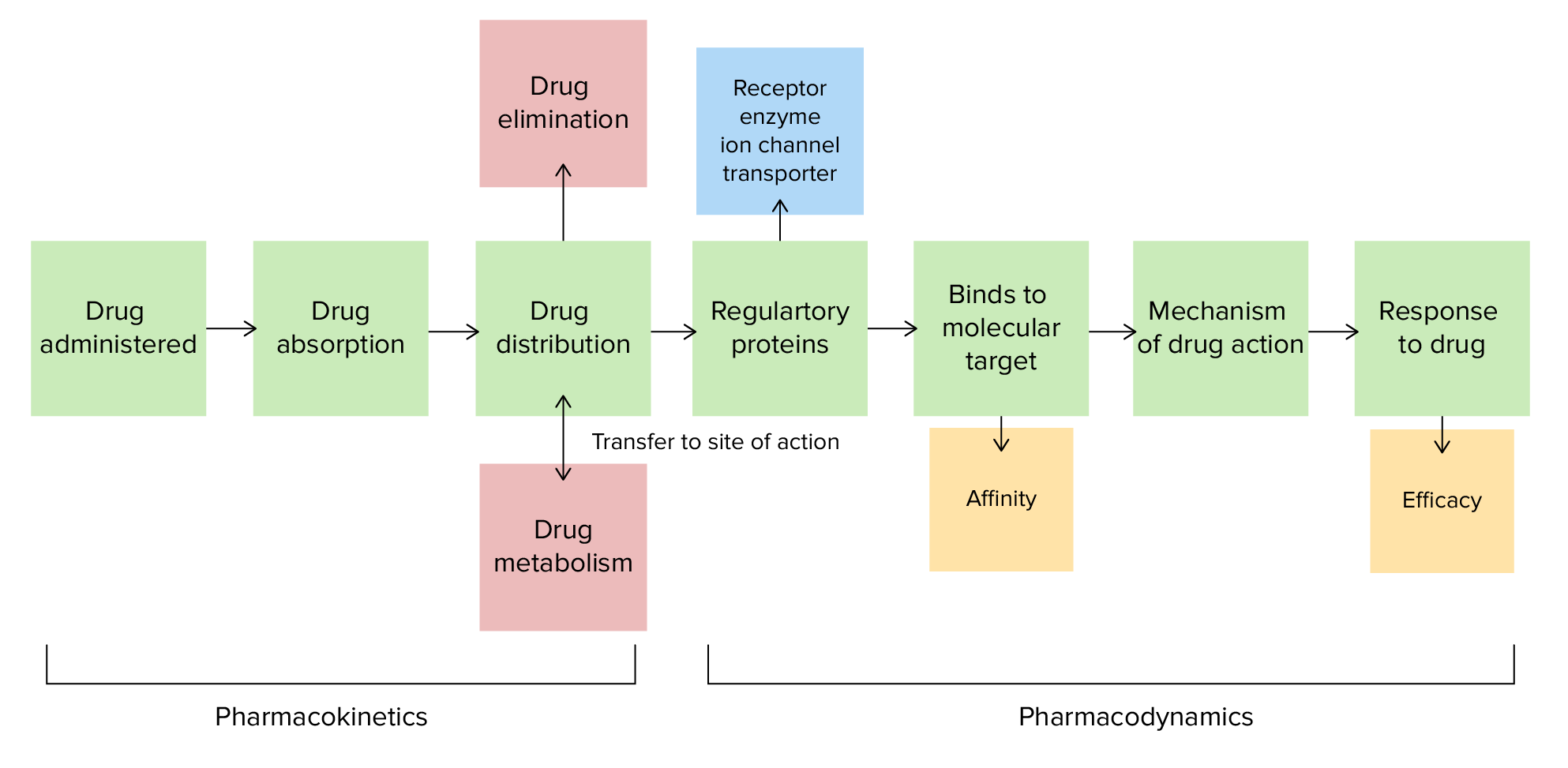
00:00 Now, we may be getting a little ahead of ourselves since we will be talking about beta-lactam antibiotics in the final slide for this module. However, it is important in the context of prodrugs and the context of the pharmacokinetic pathway. Here we have benzylpenicillin. This is a characteristic beta-lactam with a thiazolidine ring attached to it, characteristic of all penicillins. And the crucial part of this molecule, without which there is no antibiotic activity whatsoever is the beta-lactam ring. The beta-lactam ring is the cyclic amide following the square shape within that structure. One of the reasons why bioavailability is an issue with the early penicillins, such as benzylpenicillin, is that it was prone to acid catalysed, intra molecular hydrolysis facilitated by the tautomerism of that amide group so that you get the aminium ion shown there with the O- in the 6 position of the penicillin structure. This is highly nucleophillic. 01:11 What it then does, as we appreciate, is that nucleophilic attack on the strained beta-lactam ring, shown by the green arrows, which carries out an addition elimination reaction which you should remember from Module III, when we talked about carboxylic acid derivatives. 01:28 And what this does is it forms an intermediate, which then opens up that beta-lactam ring, interestingly converting it from a strained amide to an ester. 01:40 Now, I know that flies in the face of that which we discussed before when we were talking about amides being hydrolysed and converted into esters, but the reality here is that that four membered ring is highly strained. It wants to break open and via acid hydrolysis, which, in this case, takes place in the stomach which has a low pH. What effectively happens is that most of the benzylpenicillin that would be taken orally is broken down before it even has a chance to be absorbed through the gastrointestinal tract. The absence of the beta-lactam ring doesn’t just actually reduce the activity, it destroys it. There is no antibiotic activity associated with the final compound in the bottom left hand corner. So, the solution. As we’ll see a little later on, but I’ll briefly lead to you here, incorporating an R group within the 6 position on that amide which is electron-withdrawing. So, for example, a phenoxymethyl group, such as first made in the case of penicillin V, pulls the electron density away from that amide carbonyl. By pulling the density away from that carbonyl, it reduces its nucleophilicity and therefore, it does not attack the amide. And penicillin V is far more stable to acids than benzyl penicillin and was one of the first penicillins to be already bioavailable and therefore, to be given as an oral dose. Okay. Now, I’d like to talk to you about another element of drug distribution, which is trying to get a molecule into the brain. 03:16 Now, the brain or around the brain, you have the Blood Brain Barrier or BBB, for short, which is a layer of tightly packed endothelial cells that only allow small molecules in, less than 500 daltons, or to put it in another way, molecules which have a molecular weight of less than 500 grams per mole. And they have to be lipophilic in order to cross by diffusion. As you can see, the difference between a general capillary, where you have an intercellular cleft passage and you have passive diffusion, is that you have a tight junction in a brain capillary with these astrocyctic processes surrounding it. This means that it must be small and highly lipophilic to pass through the brain or else be taken up via active transport.
About the Lecture
The lecture Acid Sensitivity – Pharmacokinetics and Pharmacodynamics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
What is the main reason behind limited bioavailability of benzylpenicillin?
- The beta-lactam ring of benzylpenicillin is highly prone to acid catalyzed intramolecular hydrolysis.
- The beta-lactam ring of benzylpenicillin is highly prone to alkali catalyzed intramolecular hydrolysis.
- The beta-lactam ring of benzylpenicillin is highly prone to acid catalyzed intermolecular hydrolysis.
- The beta-lactam ring of benzylpenicillin is highly prone to alkali catalyzed intermolecular hydrolysis.
To cross the blood-brain barrier, a drug molecule should possess which of the following characteristics?
- Highly lipophilic with small molecular mass
- Highly lipophilic with high molecular mass
- Highly hydrophilic with small molecular mass
- Highly hydrophilic with high molecular mass
- Neutral with high molecular mass
Customer reviews
1,8 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
4 |
6 customer reviews without text
6 user review without text