Playlist
Show Playlist
Hide Playlist
Serine Proteases – Enzyme Catalysis
-
01 Advanced Enzymes&Kinetics1.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
00:00 Okay. Nun, Serinproteasen spalten wie gesagt Peptidbindungen. Das ist die katalytische Aufgabe, die sie erfüllen. 00:06 Sie haben eine Spezifität beim Schneiden, denn sie binden nur an bestimmte Proteine. 00:12 Sie schneiden nur die Proteine, die sie binden. Sie haben ein gemeinsames aktives Zentrum. 00:18 All die verschiedenen Serinproteasen besitzen eine dreidimensionale Konfiguration des Ortes, an dem die die Reaktion stattfindet. 00:27 Jetzt werden wir sehen, dass das wichtig ist, denn diese Konfiguration schafft die elektronische Umgebung, die für das Zustandekommen der Reaktion erforderlich ist. 00:37 Und schließlich sind Serinproteasen sehr gut untersucht. Wir verstehen also den Mechanismus ihrer Wirkung recht gut. 00:43 Werfen wir nun einen Blick auf den Mechanismus der Serinproteasen. 00:47 Ich habe hier auf dem Bildschirm ein Substrat für das Enzym dargestellt. 00:51 Dies ist eine Polypeptidkette, ein Protein, das die Serinprotease schneiden wird. 00:58 Dieser spezifische Schnitt wird hier erfolgen: zwischen dem Kohlenstoff und dem dem Stickstoff dieses Moleküls. 01:04 Und natürlich kennen Sie diese Struktur, da wir bereits in anderen Präsentationen über sie gesprochen haben. 01:08 Es ist die Position der Peptidbindung. 01:09 Auf der rechten Seite des Bildes können Sie den zentralen Teil einer Serinprotease sehen. Der zentrale Teil ist der Ort, wo die Reaktion katalysiert wird. 01:22 Jetzt wird es ein bisschen schwierig, einige dieser Dinge zu verstehen. 01:25 Daher werden Sie sehen, dass ich manchmal Bindungen und Moleküle ein wenig strecke, damit alles dann auch passt, sodass Sie es verstehen können. 01:33 Bitte verstehen Sie, dass im eigentlichen Enzym selbst diese Bindungen und Moleküle natürlich bereits besser aufgestellt sind, aber es ist schwer bei solchen Schemata, die Dinge so passend zu machen, dass Sie es verstehen. Alle Serinproteasen haben ein gemeinsames Merkmal in ihrem aktiven Zentrum. Und das gemeinsame Merkmal in ihrem aktiven Zentrum ist, dass sie alle diese drei Aminosäure-Seitenketten besitzen, die Sie hier in unmittelbarer Nähe zueinander sehen können. 01:55 Ich erinnere die Studenten immer gerne daran, dass, wenn wir so etwas sehen, es uns daran erinnert, dass die Faltung von Proteinen tatsächlich stattfindet. 02:04 Denn: dieses Serin, Histidin und die Asparaginsäure - das sind die drei Seitenketten Ketten, die wir hier sehen - liegen in der Primärstruktur nicht nahe beieinander. 02:10 Sie werden aber durch die Faltung des Enzyms physisch näher zueinander gebracht, wie wir hier sehen. 02:22 Und die Nähe zwischen diesen ist für den Anfang wichtig. 02:25 Aber was noch wichtiger ist: die Flexibilität des Enzyms mit diesen Seitenketten ist absolut notwendig für die katalytische Funktion, die stattfinden wird. 02:34 Okay. Wir stellen uns nun dieses gefaltete Enzym vor. 02:38 Der Rest des Enzyms ist gelb eingefärbt. Wir sehen jetzt gerade speziell das aktive Zentrum. In der Nähe des aktiven Zentrums befindet sich eine Stelle, an der das Protein binden wird, und das Protein, das geschnitten werden soll, interagiert mit dieser katalytischen Triade aus Serin, Histidin und Asparaginsäure. 02:56 Die Bindung des Substrats an das Enzym erfolgt an einer speziellen Stelle des Enzyms, der sogenannten S1-Tasche. 03:05 Wir haben hier also die S1-Tasche: eine Art Halbkreis, der einen Teil des Proteins festhält. 03:12 Wir können sehen, dass das Protein, das jetzt jetzt geschnitten wird, sich an der aktiven Stelle befindet. 03:17 Bei der Bindung dieses Proteins an das aktive Zentrum seheh Sie, dass der Stickstoff des Histidins einen Pfeil hat, der auf das Hydroxid-Ion weist. Wir stellen auch fest, dass der Sauerstoff der Seitenkette der Asparaginsäure einen kleinen Punkt neben dem Wasserstoff des Histidins besitzt. 03:37 Was ist hier passiert? Nun, wenn wir von der vorherigen Folie zu dieser Folie springen, können wir sehen, dass sich die Form des Enzym ein kleines bisschen verändert hat. 03:48 Die Bindung des Substrats - und denken Sie daran, dass die Bindung von Substrat Enzyme verändert - hat das Enzym ein klein wenig verändert. 03:56 Sodass sich die Entfernung der Seitenkette der Asparaginsäure zur Histidin-Seitenkette verändert hat. 04:03 Das ist sehr wichtig. Bei der Asparaginsäure hier besitzt der Sauerstoff eine negative Ladung, und die negative Ladung hat sich ein wenig näher an den Ring des Histidins heranbewegt, wie hier gezeigt. 04:15 Durch diese kleine Handlung wird die elektronische Konfiguration des Histidinringes verändert. 04:23 Und diese Veränderung führt dazu, dass der Stickstoff sich nun ausstreckt und dieser sich wiederum das Wasserstoffatom des Serins schnappt, okay? Diese winzige Formänderung, die bei der Bindung des Enzyms stattfindet, setzt also den Prozess in Gang, durch den die Reaktion stattfinden wird. 04:41 Wir sehen hier, dass die S1-Tasche all dies ermöglicht hat. 04:46 Ich sollte hinzufügen, dass die S1-Tasche die Spezifität des Enzyms bestimmt. 04:54 Die S1-Tasche bindet nicht alles. 04:57 Sie bindet an spezifische Proteine mit bestimmten Sequenzen. 05:03 Sehr, sehr wichtiges Konzept. Wenn sie diesen spezifischen Dingen nicht begegnet, wird sie sie nicht binden und wenn sie sie nicht bindet, gibt es natürlich keine Reaktion. 05:10 Am Ende wird dieser Prozess nicht stattfinden. Okay. Die leichten strukturellen Veränderungen sind also passiert und wir sehen nun das Ergebnis hiervon, das sich langsam bemerkbar macht. Die Moleküle haben sich einander angenähert. Die elektronische Umgebung hat sich zu diesem Zeitpunkt definitiv verändert. 05:29 Und wir sehen nun, dass das Proton, das am OH des Serins war, sich dem Stickstoff des Histidinrings angeschlossen hat. Dies ist der erste Schritt in diesem katalytischen Prozess. Eigentlich der zweite Schritt, wenn wir die Bindung des Substrats mit dazu zählen. 05:45 Die Erzeugung des Sauerstoffs mit einer negativen Ladung am Zipfel des Serins hat eine grundlegende Bedeutung für das Zustandekommen dieser Reaktion. 05:54 Wir nennen diesen negativ geladenen Sauerstoff am Serin ein Alkoxid-Ion. Okay? Dieses Alkoxid-Ion am Serin ist außerordentlich reaktionsfreudig. 06:04 Es wird gleich ordentlich Geschäfte machen. 06:07 Jetzt haben wir die S1-Tasche etwas gedehnt, um uns daran zu erinnern, dass wir wieder alles näher zusammenbringen. Und das ist wichtig, denn das Alkoxid-Ion sucht nach einem Bindungspartner. Es ist auf der Suche nach ein Atomkern. Das nennen wir ein Nukleophil. 06:25 Und der Kern, nach dem es hier sucht ist dieser Kohlenstoff. Das wird veranschaulicht durch den Pfeil vom negativ geladenen Sauerstoff zum orangefarbenen Kohlenstoff hinunter. 06:35 Es findet also ein sogenannter chemischer Angriff statt, ein nukleophiler Angriff, der an diesem Kohlenstoff stattfindet. 06:43 Die Elektronen, die doppelt an den Sauerstoff gebunden sind, werden umgelagert, wie der Pfeil zeigt. 06:51 Und im nächsten Schritt des Prozesses wird es zu einer Umstrukturierung des Moleküls kommen. 06:58 Okay? Wir sind also von dieser Anordnung zu dieser hier gekommen. Beachten Sie, dass wir ein Kohlenstoffatom mit einer Doppelbindung an einem Sauerstoff hatten und jetzt ist es ein Kohlenstoffatom mit einer Einfachbindung am Sauerstoff. 07:11 Dieses Molekül ist chemisch instabil. 07:12 Es ist chemisch instabil und um ein chemisch instabiles Molekül müssen wir uns kümmern, ansonsten kommt es zu Problemen. 07:21 Nun, das Enzym besitzt eine weitere Tasche, die sich um solch instabile Moleküle kümmert: das sogenannte Oxyanion-Loch. 07:28 Und das Oxyanion-Loch hilft dabei, das Molekül zu stabilisieren. 07:33 Das ist ziemlich cool. 07:36 Okay? Es wird ohne Probleme stabilisiert. Und hier passiert folgendes, wie Sie sehen: Der Stickstoff in Blau greift nach oben und schnappt sich den Wasserstoff der ursprünglich von der Histidin-Seitenkette festgehalten wurde, okay? Dieses Zwischenprodukt, das sich im Oxyanion-Loch befindet, nennen wir einen Tetraeder, okay? Tetraeder kennen wir aus der organischen Chemie. 08:00 Charakteristisch sind diese vier Bindungen des Kohlenstoffs, die Sie hier sehen können, okay? Die Peptidbindung zwischen dem Kohlenstoff und dem Stickstoff, wird gebrochen, weil der Stickstoff sich den Wasserstoff schnappt. 08:13 Hier hat sich der Stickstoff den Wasserstoff geschnappt. Das Abgreifen des Wasserstoffs vom Histidin führt dazu, dass die Bindung zwischen dem Kohlenstoff und dem Stickstoff bricht. 08:23 Wir haben also die Peptidbindung gebrochen, und ein Teil des des Proteins - der hier blau markiert ist - ist jetzt frei und kann sein Ding machen. Es ist losgelöst. 08:32 Es gibt nichts, das es an das Enzym binden würde, also löst es sich los und existiert weiter. 08:35 Wir haben hier tatsächlich den ersten Teil der Reaktion durchlaufen. 08:42 Und diese Teilreaktion nennen wir die schnelle Teilreaktion, okay? Der andere Teil des Proteins ist an das Serin gebunden. 08:51 Es ist physisch mit Serin verbunden. Zu diesem Zeitpunkt ist es eine kovalente Bindung. 08:55 Diese kovalente Bindung muss nun gebrochen werden, damit der andere Teil des ursprünglichen Proteins freigesetzt werden kann. 09:04 Und genau das passiert im langsamen Teil der Katalyse. 09:08 Der langsame Teil der Katalyse hat ungefähr die gleiche Anzahl von Schritten wie der schnelle Teil. 09:14 Aber es müssen noch andere Dinge passieren, zum Beispiel die Bewegung von Wasser in das aktive Zentrum, damit dieses Peptid freigesetzt werden kann. 09:23 Nun, wir sehen, dass das hier passiert. Wasser hat sich nun physisch in das aktive Zentrum hineinbewegt. Dort befindet sich jetzt ein Wassermolekül. Und dieser Prozess, den wir gesehen haben, wobei der Stickstoff am Histidin ein Proton aufnimmt, wird sich wiederholen. 09:36 Wir sehen es hier. Wir sehen den Pfeil vom Stickstoff des Histidins, der auf den Wasserstoff des Wassers zeigt. 09:43 Er nimmt also diesen Wasserstoff anstatt des Wasserstoffs von vorhin, also der, der ursprünglich am Serin war und jetzt nicht mehr da ist. 09:49 Was in diesem Prozess passieren wird, ist, dass wir jetzt wieder einen aktivierten Sauerstoff haben wie bei dem Alkoxid-Ion, außer dass es hier ein Hydroxid-Ion ist. 10:00 Wir werden einen aktivierten Sauerstoff haben, der einen nukleophilen Angriff auf Kohlenstoff vornimmt, wie wir es zuvor gesehen haben. 10:07 Es gibt also einen nukleophilen Angriff, der der sich im Laufe des Prozesses vollzieht. 10:13 Hier ist der Angriff des Hydroxids und schauen Sie, was passiert. Wir sehen, dass die Elektronen des Sauerstoffs sich neu anordnen. 10:22 Wir schaffen ein tetraedisches Zwischenprodukt, wie zuvor. Und jetzt gibt es das Oxyanion-Loch, das dieses Zwischenprodukt stabilisiert. 10:33 Wir sehen nun, dass der Sauerstoff den Wasserstoff an dieser Gruppe angreift ihn wegreißt, genau wie beim ersten Peptid. 10:42 Wenn das geschieht, wird das Molekül freigesetzt. Wir sehen also, wie die zweite Hälfte der Polypeptidkette freigesetzt wird und außerdem, wie das Enzym in seinen ursprünglichen Zustand zurückversetzt wird. So wie es vorher war. 11:03 Der Zyklus ist nun abgeschlossen. Es sind etwa 10 Schritte, die ich hier beschrieben habe und das Wichtigste daran ist, dass das Enzym in einem bestimmten Zustand begonnen hat. 11:13 Es durchlief eine Übergangsphase und kehrte dann in den ursprünglichen Zustand zurück. Ganz ähnlich wie der Prozess, den ich bereits beschrieben habe aber jetzt haben Sie es in mechanistischer Hinsicht gesehen. 11:22 Als wir das Bild der Reaktion gesehen haben, sahen wir die verschiedenen Zustände, die Sie auf dem Bildschirm sehen.
About the Lecture
The lecture Serine Proteases – Enzyme Catalysis by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
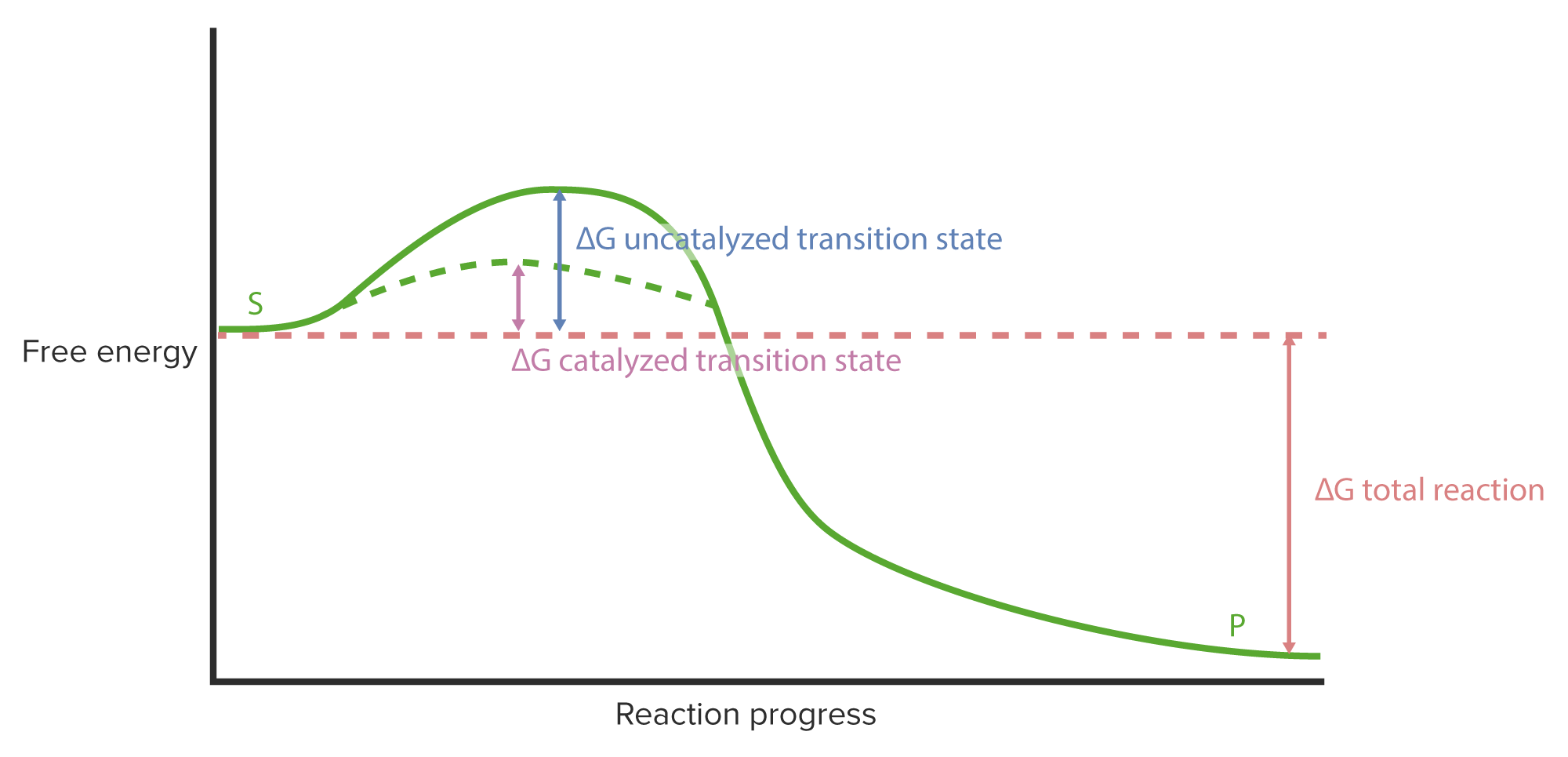
Why is enzyme catalysis so effective?
- It lowers the energy of activation of a reaction.
- It changes the Gibbs free energy change of a reaction.
- Enzymes have constant, unmodulated activity.
- It allows reactions to go forward.
Which of the following is true of serine proteases?
- They use serine for catalysis in their active sites.
- They cut target proteins at serine residues.
- They bind serine during catalysis.
- They are misnamed because they do not involve serine at all.
The catalytic triad of the serine protease is composed of which of the following?
- Serine, histidine, and aspartic acid
- Serine, glutamic acid, and histidine
- Serine, glycine, and aspartic acid
- Serine, lysine, and histidine
- Serine, alanine, and selenocysteine
Which of the following site allows for the binding of an unstable intermediate during the proteolytic reaction of serine proteases?
- Oxyanion hole
- Active site
- Allosteric site
- S1 pocket
- Hydroxide/alkoxide pocket
Which of the following determines the specificity of the enzyme, serine protease?
- S1 pocket
- Oxyanion hole
- Active site
- Allosteric site
- Alkoxide/hydroxide pocket
Which of the following acts as a nucleophile during the catalytic action of a serine protease enzyme?
- Alkoxide ion produced on serine.
- N+ of histidine ring
- H+ of serine
- O- of aspartic acid
Which of the following is true about the mechanism of action of serine proteases?
- Aspartic acid and histidine play important roles.
- An electrophilic attack is at the core of the reaction mechanism.
- The slow step is the first one.
- The alkoxide ion forms on aspartic acid.
Which of the following is true of the S1 pocket of a serine protease?
- It determines the binding/cutting specificity of the enzyme.
- It stabilizes an unstable tetrahedral intermediate.
- It is the location of the catalytic triad.
- It requires serine for binding.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
3 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Attaractively and well explained video of serine protease catalytic mechanism that I have ever seen...Thank you..????
It is really helpful. I appreciate your kind efforts. Thanks verh much.
He simplified the concepts and illustrated by a very nice way