Playlist
Show Playlist
Hide Playlist
Saccharides – Simple Carbohydrates
-
06 Basic SimpleCarbohydrates V2.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
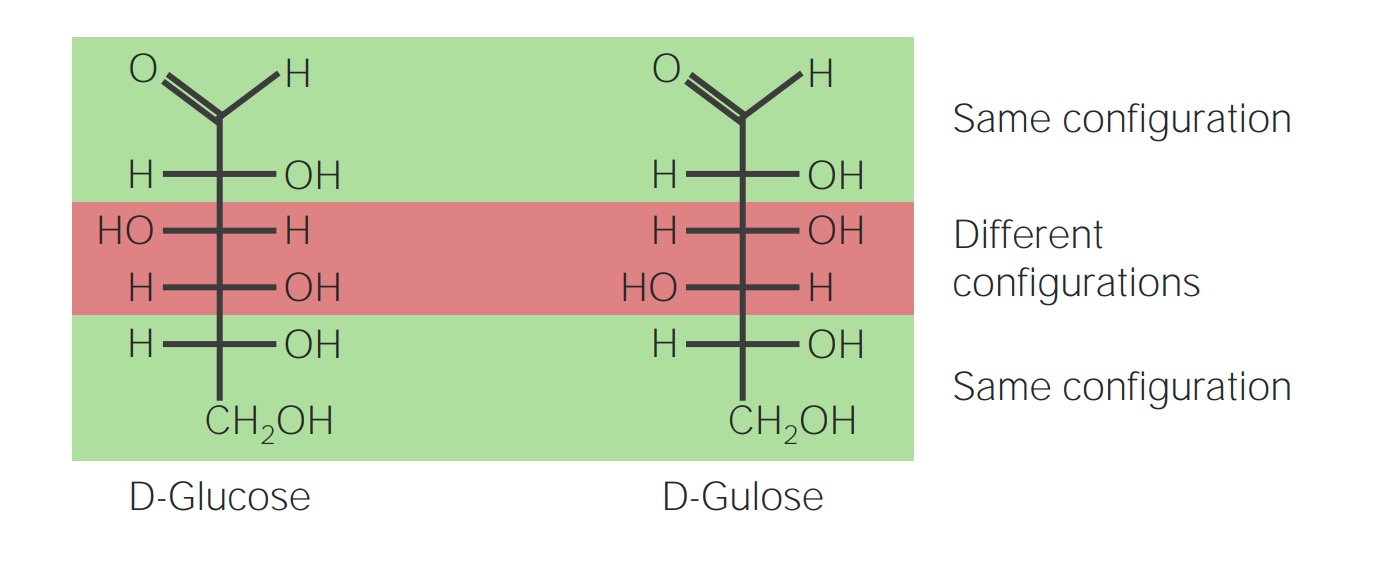
00:01 Kohlenhydrate sind Moleküle, deren Name wörtlich 'Hydrate aus Kohlenstoff' bedeutet. Wie wir sehen werden, wenn ich über die Strukturformeln für diese spreche, ist das buchstäblich wahr. In dieser Vorlesung werde ich die Nomenklatur der Kohlenhydrate besprechen, die Strukturen, die sie haben, die Art und Weise, wie sie Ringstrukturen bilden, und chemische Modifikationen, die vorgenommen werden, um sie zu metabolisieren. 00:24 Diese Folie zeigt eine Darstellung von Monosacchariden, d.h. Kohlenhydraten, die nur ein einziges Molekül enthalten und somit Einfachzucker genannt werden. Die allgemeine Formel, mit der wir diese beschreiben können, ist Cx(H2O)x, wobei x eine ganze Zahl ist. Im Fall des Zuckers Glukose zum Beispiel, ist die Struktur C6H12O6. Wir sehen, dass dies auch für für Fruktose und Galaktose zutrifft. Ribose ist ein Zucker mit fünf Kohlenstoffen und einer Gesamtstruktur von C5H10O5, Glyceraldehyd C3H6O3. In jedem Fall haben wir Hydrate aus Kohlenstoff. 01:04 Disaccharide sind Kohlenhydrate, die zwei Zuckermoleküle enthalten. Wenn wir also zum Beispiel Glukose nehmen und Fruktose hinzufügen, erhalten wir das Disaccharid Saccharose. 01:15 Wenn wir Glukose nehmen und ihr Galaktose hinzufügen, erhalten wir den Zucker, der als Laktose bekannt ist. Oder wenn man zwei Glukosen zusammenfügt, erhält man den Zucker, der als Maltose bekannt ist. 01:26 Polysaccharide sind Kohlenhydrate, die viele, viele Zucker enthalten, und normalerweise ist der Zucker in dem Polysaccharid immer derselbe. 01:39 Zu den Polysacchariden gehören Moleküle wie Zellulose, Glykogen, Amylose, Amylopektin und Chitin und in jedem Fall ist jedes dieser Polysaccharide ein Polymer aus denselben sich wiederholenden Zuckereinheiten.
About the Lecture
The lecture Saccharides – Simple Carbohydrates by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which two monosaccharides are components of lactose?
- Glucose and galactose.
- Two glucose molecules.
- Glucose and fructose.
- Two fructose molecules.
- Fructose and galactose.
Customer reviews
4,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
1 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
The following are the errors in the information you provided: 00:24 The general formula for monosaccharides is Cx(H2O)x, where x is equal to the number of carbon atoms in the monosaccharide. For example, the structural formula for glucose is C6H12O6, which means that it has six carbon atoms and six water molecules. 01:15 The structural formula for lactose is C12H22O11. This means that it is a disaccharide made up of two monosaccharides, glucose and galactose. 01:39 Polysaccharides are polymers of monosaccharides, but they do not necessarily have to have the same repeating sugar unit throughout. For example, amylopectin is a polysaccharide made up of glucose molecules, but it has a branched structure. Here is a corrected version of the information you provided: Carbohydrates are molecules whose name literally means hydrates of carbon. This is because monosaccharides, the simplest carbohydrates, have a ratio of one carbon atom to two hydrogen atoms to one oxygen atom, the same as water. The general formula for monosaccharides is Cx(H2O)x, where x is equal to the number of carbon atoms in the monosaccharide. For example, the structural formula for glucose is C6H12O6, which means that it has six carbon atoms and six water molecules. Disaccharides are carbohydrates that are made up of two monosaccharides. They are formed by a glycosidic linkage between the two monosaccharides. Disaccharides have the general formula C12H22O11. Examples of disaccharides include sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (glucose + glucose). Polysaccharides are carbohydrates that are made up of many monosaccharides. They are formed by glycosidic linkages between the monosaccharides. Polysaccharides have the general formula (C6H10O5)n, where n is the number of monosaccharides in the polysaccharide. Examples of polysaccharides include cellulose, starch, glycogen, and chitin. Polysaccharides do not necessarily have to have the same repeating sugar unit throughout. For example, amylopectin is a polysaccharide made up of glucose molecules, but it has a branched structure.
Awesome job, I wish you were my teacher at my university! So clear!