Playlist
Show Playlist
Hide Playlist
Respect for Persons – Ethical Principles in Human Subjects Research
-
Slides Respect for Persons Ethical Principles in Human Subjects Research.pdf
-
Download Lecture Overview
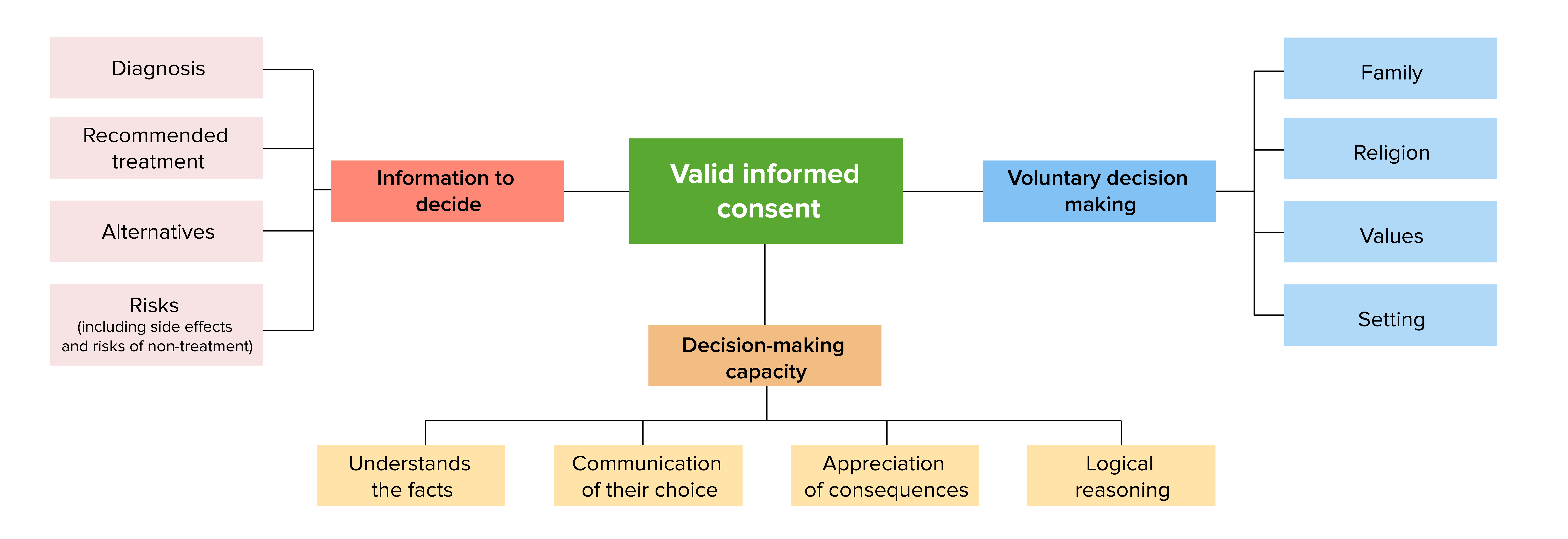
00:01 So let's look a little more deeply at each of these principles. 00:05 So respect for persons, as I said, starts with voluntariness, meaning that there's any freedom from coercion or undue influence, that the person is freely choosing to be a research participant, and that there's then being informed consent process. 00:21 So this is allowing the individual to control whether or not they want to enroll in research. 00:27 And they're basing that on their own values, their own interests, their preferences and the information that's been given to them by the researcher. 00:36 Now, in some context, there may be cultural mores that would say a community leader also has to give permission for the members of that community to participate in the research. 00:48 So you have to know what the particular locale is and what the culture is to determine how you're going to demonstrate respect for persons. 00:59 It also means that those that have diminished capacity, or sometimes we might even consider, you know, children as research participants, they will need to have someone to speak on their behalf. 01:08 So they need a proxy decision maker to decide whether or not the research is in that person's best interests. 01:17 And, you know, another aspect of respect for persons and voluntariness is this idea that research subjects can decide to withdraw from a study at any point so they can decide up front whether they want to join, but once they're a research participant, once they're in the research, they can decide to withdraw that's part of voluntariness. 01:37 Another aspect of respecting a person as a research participant is deciding how you're going to share results with them about what you found in the research, especially if it has implications for their health or well-being. 01:51 There might be an obligation of the researcher as a sign of respect to share results with that person. 01:58 So they can have something that's actionable. 02:00 The core of respect for persons is going to be informed consent. 02:04 So similar when we're thinking about clinical medicine, the need for a process to give information and have a person make a decision for themselves, that information should be should be given in understandable language. 02:17 And when we're thinking about research in particular, the top of the list, the thing that they need to be clear about is that this is research. 02:25 This is not clinical care. 02:27 There's a particular hypothesis, there's a particular research question. 02:32 That's the purpose of their joining this study is for research aims. 02:37 They need to understand what the purpose of the research is what you're trying to understand, what the knowledge that you're trying to seek in the research study is, what are going to be the procedures involved, especially from their perspective, as a research participant, what are the things that they need to do as a research participant. 02:57 They need to be aware of the potential risks, the potential benefits, and whether there are alternatives to their participation as a research participant, they might decide, well, they just want to seek clinical care, and not engage in this clinical research project. 03:13 Another aspect of informed consent, so usually, we think about that, you know, at the time of enrollment, but another aspect is, as the study is underway, and the researchers are learning new information, there might be times where that information becomes irrelevant to that ongoing participation by the research subject. 03:34 So that information also is part of what's given to the person, especially if it might be something that affects their condition, affects how the study design is, they need to be given that information in order to consent to ongoing participation or to say, well, I need to seek medical care for new information that you've learned as a result of the research.
About the Lecture
The lecture Respect for Persons – Ethical Principles in Human Subjects Research by Mark Hughes, MD, MA is from the course Clinical Research Ethics.
Included Quiz Questions
What is the core research principle of respect for persons?
- Informed consent
- Individual power
- Proxy decision-maker
- Sharing the results of research
- Withdrawal from research
Which of the following should NOT be discussed during the informed consent process?
- Members of the institutional review board
- Purpose of research
- Procedures involved
- Risks
- Alternatives
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |