Playlist
Show Playlist
Hide Playlist
Receptor Tyrosine Kinase Pathways – Second Messenger Systems
-
Slides 10 SecondMessengerSystems CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
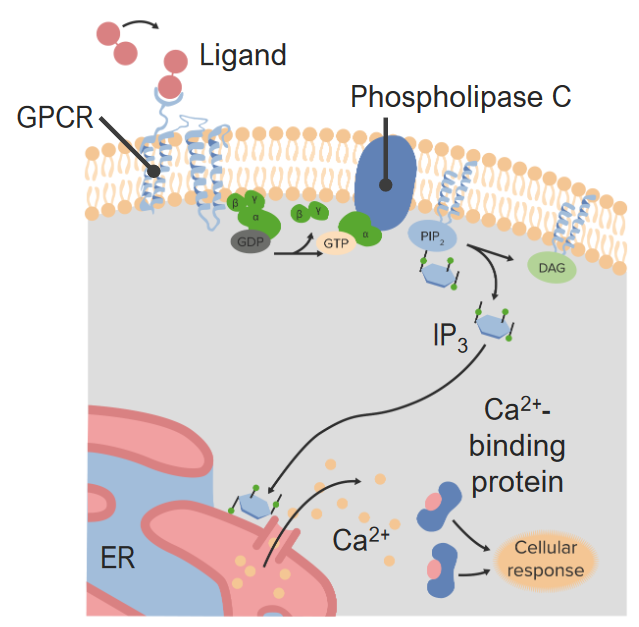
00:00 Lasst uns einen Moment innehalten und überlegen, was eine Proteinkinase ist. Eine Proteinkinase ist ein Enzym, das andere Proteine phosphoryliert. Manchmal sind diese Proteinkinasen intrazelluläre Proteinkinasen. In anderen Fällen, kann die Proteinkinase in die Zellemembran eingebettet sein. 00:26 Rezeptor-Tyrosin-Kinasen sind ein perfektes Beispiel für diese eingebetteten Proteinkinasen. 00:35 Hier haben wir also ein Protein, das von einer Proteinkinase phosphoryliert wird. 00:42 Erinnern Sie sich daran, daß die Proteinkinase ihre Phosphate entweder von ATP oder von GTP nimmt. 00:50 In unseren Beispielen nimmt sie es von G-Proteinen. Es wird ADP gebildet. Das Phosphat ist dann jedoch direkt an dem Protein dran. Die Proteinkinase ist also ein Enzym, das jedes andere Protein phosphoryliert. 01:05 Wir können auch eine Dephosphorylierung haben, die die Prozesse inaktiviert. In der letzten Vorlesung haben wir uns angesehen, wie wir Dinge durch Phosphorylierung einschalten oder ausschalten können. 01:18 Zum Beispiel kann ein Protein eine Proteinkinase phosphorylieren und damit eine Kaskade aktivieren. 01:25 Oder ein Protein kann mit einer Proteinkinase phosphoryliert werden. Dieses phosphorylierte Protein kann vielleicht einen der Zwischenschritte nehmen, ihn stoppen und in die Kaskade eingreifen. Ähnlich wie das Herausnehmen eines der Feuerwehrleute, so dass er nicht seine Arbeit machen kann. Eine andere Sache, die passieren kann, ist, dass wir diese Protein, das phosphoryliert wurde recyclet wird. Es wird dann dephosphoryliert. 01:51 Normalerweise überträgt das phosphorylierte Protein sein Phosphat auf ein anderes Protein und dann wird dieses wiederum zu einem anderen Protein übertragen. Und so weiter und so fort, die ganze Kaskade hinunter, bis wir tatsächlich unser vollwertiges zelluläre Reaktion haben. Erinnern Sie sich, dass ich erwähnt habe, dass Rezeptor Tyrosinkinasen an der allgemeinen Zellreaktion beteiligt sind. 02:09 Das Alltagsleben einer Zelle. Dinge wie Zellzyklus und Zellwachstum. Zellwanderung von Ort zu Ort. Zellstoffwechsel, Zellproliferation und viele Prozesse der Wachstumsfaktorregulation. 02:26 Wachstumsfaktoren wirken also oft über Rezeptor Tyrosinkinasen, um ihre Wirkung in Wachstum und Entwicklung zu entfalten. 02:32 Bevor wir uns mit den Rezeptoren beschäftigen, wie sie im Detail funktionieren und was in der Kaskade passiert, schauen wir uns die Anatomie einer Rezeptor-Tyrosin-Kinase an. 02:44 Was Sie hier sehen, sind zwei Rezeptortyrosinkinasen. Sie haben jeweils eine einzige Transmembrandomäne, der alphahelische Teil. Sie haben auch eine Rezeptor, den Ligandenbindungsdomäne, an der Außenseite der Zelle. 03:01 Und im Inneren der Zelle befindet sich das eigentliche Rezeptor-Tyrosin-Kinase-Protein. Hier ist also die Proteinkinase mit dem Rezeptor verbunden, der sich an der Außenseite der Zelle befindet, anstatt einen intrazelluläre Proteinkinase, mit der wir uns zuvor befasst haben. Sobald also ein Ligand am Rezeptor der Rezeptor-Tyrosinkinase bindet, kommt es zur Dimerisierung. 03:28 Das heißt, diese beiden Untereinheiten kommen zusammen. Wenn sie zusammenkommen, phosphorylieren sie. 03:35 Erinnern Sie sich, dass der Teil, der in die Zelle hineinragt, der eigentliche Proteinkinase-Teil ist. Und weil er eine Proteinkinase ist, die Phosphate von ATPs nehmen kann, kann sie diese an die andere Rezeptor-Tyrosin-Kinase anhängen. 03:49 Auf diese Weise diamerisieren sie, sie verbinden sich also miteinander. Die Phosphotyrosin-Regionen, die Tyrosine mit den Phosphate an der Seite, dienen als Andockstellen für andere Proteine, die an der Signaltransduktion der Kaskade beteiligt sind. 04:06 Die Reaktion, die wir von einer Tyrosinkinase-Diamerisierung erhalten, ist abhängig von der Art der beteiligten Antwortproteine. Meistens handelt es sich dabei um Enzyme. 04:20 Damit Proteine oder Enzyme an die Phosphotyrosine andocken können, benötigen wir oft Andockproteine. Andockproteine helfen also anderen Enzyme an die Phosphotyrosin-Stellen anzudocken. 04:36 Zusätzlich zu den Andockproteinen benötigen wir manchmal Adapter. Wenn man zum Beispiel Strom in einem fremden Land aus der Steckdose haben möchte benötigt man manchmal einen Adapter. Hier wollen wir mit dem Adapter sicherstellen, dass direkt an diesen speziellen Zacken oder Phosphate, die aus den Phosphotyrosinen heraus ragen, angedockt werden kann. Sobald diese beiden diamerisiert sind und die Proteinkaskade aktiviert ist, gibt es eine Vielzahl verschiedener Möglichkeiten das passieren können. In Kürze werden wir uns ein paar großartige Beispiele für eine Kaskade anschauen, die aus der Aktivierung der Rezeptor-Tyrosinkinase resultieren. Im Fall von Insulin bindet es also an die extrazelluläre Rezeptordomäne. Und dann aktiviert es die Rezeptor-Tyrosin-Kinasen. Sie autophosphorylieren einander. Dann haben wir das Insulinreaktionsprotein, ein Andockprotein, das an die Phosphat-Zacken der Rezeptor-Tyrosinkinase bindet. Danach wird das Phosphate an andere Proteine weitergegeben, die die dann zur Aktivierung der Glykogensynthase führen. 05:51 Es werden also eine ganze Reihe von Proteinen phosphoryliert. Das führt im Wesentlichen zur Transkription und Übersetzung von Dingen, die Glykogensynthase bilden. Diese Glykogensynthase klebt dann Glukosemoleküle zusammen, um Glykogen zu bilden. In diesem Sinne reduziert das Insulin also den Zucker, der frei herumschwimmt und bereit für den Stoffwechsel wäre und verwandelt ihn in Glykogenmoleküle, um dadurch den Blutzucker zu senken und den Kohlenhydratspeicher aufzufüllen. Wie erfolgt die Verstärkung dieser Signale tatsächlich? Es gibt ein großartiges Beispiel dafür, die mitogenaktivierte Proteinkinase-Kaskaden oder MAP-Kaskaden. Hier werden Kinasen durch eine Reihe von Proteinkinasen aktiviert. 06:42 Diese mitogenen Kinasen werden also schrittweise aktiviert. Und in dem Diagramm, das wir vorhin gesehen haben, können Sie sehen, dass jeder Schritt auf dem Weg das Potenzial zur Verstärkung des Signals birgt. 06:54 Zunächst einmal haben wir ein Modul von Proteinkinasen. Und wir werden gleich sehen, wie diese miteinander verbunden sind. 07:02 Sie werden sich gegenseitig phosphorylieren. Hier haben wir also Beispiel für eine Phosphorylierung, die durch jede dieser aktivierenden Kinasen weitergegeben wird. Es ist nicht wichtig genau zu wissen, welche das sind, sondern wir müssen nur das allgemeine Schema wissen, wie das Phosphat weitergeben wird und wie das Signal verstärkt werden kann. So dass am Ende mehr Proteinkinasen und damit mehr Phosphorylierungen entstehen. 07:27 Damit haben wir schlussendlich die Phosphorylierung der MAP-Kinase. Und wie wir gleich sehen werden, spielt die MAP-Kinase eine besondere Rolle bei der Verknüpfung von zellulären Antworten. Also noch einmal, wenn wir uns jeden Schritt dieser mitogenaktivierten Kinasekaskade anschauen, können wir sehen, dass dieses Signal weiter und weiter und weiter verstärkt werden kann, bis wir schließlich eine zelluläre Antwort erhalten. Ich erwähnte, dass diese in einem Modul gruppiert werden. Das liegt daran, dass sie Gerüstproteine genannt werden. Sie sind im Wesentlichen ein Protein, das Proteinkinasen enthält, die an dieser Mitogen-aktivierenden Kinase-Kaskade beteiligt sind. 08:10 Sonst würden diese frei in der Zelle herumschwimmen und es wäre wirklich schwer, sie zusammen zu bekommen. Deshalb halten Gerüstproteine Gruppen von verwandten Proteinen in einem Signalweg zusammen, so dass die Reaktion ziemlich lokal von statten gehen kann. Die zelluläre Reaktion könnte zu vielen möglichen Zielen führen. Wir könnten die Transkriptions- und Translationsrate erhöhen, oder die Phosphorylierung von Transkriptionsfaktoren, damit sie an die DNA andocken können und so zur Aktivierung des Prozesses beitragen. 08:45 Wir werden uns einige dieser spezifischen Proteine in einer späteren Vorlesung anschauen. Aber die Aktivierung der Genexpression ist einer der Schlüssel zu diesen Rezeptor-Tyrosinkinase Wegen. Molekulare Schalter können auch Rezeptor-Tyrosin-Kinasen zu MAP-Kinase-Kaskaden verbinden. Werfen wir einen Blick auf ein Beispiel für einen molekularen Schalter. 09:10 Diese Schalter können externe Signale mit internen Transduktionswege verbinden. Diese werden manchmal unterbrochen , was dann zu Krebs führen kann. Wenn wir die Zellteilung und die Kontrolle des Zellzyklus betrachten, können wir einen dieser Mechanismen sehen. Rezeptor-Tyrosin-Kinasen werden oft in Verbindung mit Proteinkinase-Kaskaden durch Aktivierung molekulare Schalter gebracht. Hier ist ein Beispiel für einen molekularen Schalter. 09:44 Oft werden die molekularen Schalter durch externe Zellsignale an einem anderen Rezeptorort aktiviert. 09:51 Wir werden gleich sehen, wie sie zusammenkommen. Wir haben also dieses externe Signal, das in diesem Fall die Guanin-Austauschfaktoren aktiviert. Guanin-Austauschfaktoren nehmen GTP (Guanintriphosphat) und nehmen hier die Phosphate ab und übertragen sie auf ein anderes Protein. In diesem Fall handelt es sich um RAS. RAS wird durch die Übertragung von GTP auf sich selbst aktiviert. Und es wird inaktiviert, wenn das GTP entfernt wird. 10:28 Die Klasse der RAS-Proteine ist zur Zeit ein sehr aktives Forschungsgebiet der Forschung und hier können wir sehen, wie es genau als molekularer Schalter funktioniert. Also, ein anderer Rezeptor aktiviert Guanin-Austauschfaktoren, die dazu beitragen, das Protein RAS zu phosphorylieren oder dephosphorylieren. Wenn wir also das ATP entfernen, wird RAS deaktiviert. Und wenn wir das GTP hinzufügen, wird RAS aktiviert. Hier habe ich mal zusammengefasst, wie ein Rezeptortyrosinkinase-Weg funktionieren könnte, einschließlich der Rezeptortyrosinkinase selbst, der MAP-Kaskade, und unser molekularer RAS-Protein-Schalter. Wir haben also RAS als Schalter in der Mitte, der die Rezeptor-Tyrosinkinase zur MAP-Kinase-Kaskade verbindet. Das ganze beeinflusst dann die Art der zellulären Reaktionen, die wir durch eine Vielzahl verschiedener Transkriptionsfaktoren aktivieren. 11:42 Die Reaktion, die wir erhalten, hängt wiederum davon ab, welche Proteine aktiviert werden, was ziemlich komplex ist, denn das hängt von allen möglichen anderen Rezeptoren ab, die in die Zelle gelangen. Also noch einmal: Wie können die Zellen wirklich entscheiden, was sie tun wollen? Nun, sie müssen sich danach entscheiden, was die DNA 'sagt', welchen Leuten im Raum sie zuhören wollen. Erinnern Sie sich mal an die Analogie eines Raums voller Menschen. 12:10 Es ist sehr laut und hektisch. Wie kann man mit den Leuten neben einem kommunizieren? Die Zelle muss wählen, welche Signale sie hört. Je nachdem, welche Rezeptoren in der Zellmembran vorhanden sind. 12:23 Es gibt es also mehrere Ebenen für die Modulation dieser Signaltransduktion Systeme. Je mehr Akteure es gibt, desto komplexer ist es. Tatsächlich ist es für mich oft überraschend, dass irgendetwas davon überhaupt funktioniert.
About the Lecture
The lecture Receptor Tyrosine Kinase Pathways – Second Messenger Systems by Georgina Cornwall, PhD is from the course Cellular Structure.
Included Quiz Questions
Which of the following is involved in the receptor tyrosine kinase (RTK) cascade? Select all that apply.
- Dimerization of two protein kinase receptor subunits
- Autophosphorylation
- Docking proteins and adapter proteins
- G-protein kinases
- Dephosphorylation of the dimerized protein kinase subunits
Which of the following is NOT true regarding the RTK system?
- Insulin binding to the insulin-related RTK system leads to the immediate separation of two RTK subunits to stop the propagation of the signal cascade.
- RTKs are membrane-embedded kinase proteins that play an important role in general cell functions such as cell cycle, growth, migration, and metabolism.
- Maintenance of blood glucose levels by insulin involves the RTK pathway.
- Binding of a ligand to the extracellular receptor domains causes autophosphorylation of the intracellular domains at the serine, threonine, and tyrosine residues.
- RTKs are composed of two RTK monomer units, each with a single hydrophobic α-helical transmembrane domain, which dimerize upon binding of the ligand to the receptor sites.
Which of the following results from activation of the insulin RTK signaling pathway?
- Phosphorylation of proteins in the signaling cascade will upregulate the transcription factors that increase the production of glycogen synthase.
- Denaturation of proteins will increase the destruction of GLUT channels, preventing the entry of glucose into cells.
- Synthesis of docking sites on RTKs that downregulates transcription factors for glycogen synthase
- Dephosphorylation of proteins that cleave molecules of glucose from glycogen, increasing blood glucose levels
- Upregulation of cortisol receptors
Which of the following statements best describes scaffold proteins?
- They are involved in the organization of the signaling cascade proteins/components into a highly ordered macromolecular complex for efficient signal transduction.
- They are active enzymatic components of the signaling cascade.
- They are involved in the transfer of phosphate groups from kinases and phosphatases to substrate proteins to switch them "on" or "off."
- They directly deal with the reception of an external signal to stimulate cellular responses.
- They act as molecular switches.
Which of the following is INCORRECT about molecular switches?
- Molecular switches link intracellular signals to external transduction pathways.
- Ras acts as a connecting link between receptor tyrosine kinase and mitogen-activated protein kinase.
- Ras proteins are activated and deactivated by guanine nucleotide exchange factors and GTPase activating proteins, respectively.
- The Ras system regulates the cell cycle and cell division by activating the mitogen-activated protein kinase cascade.
- The breakdown of intramolecular switches may lead to cancers due to deregulated cell-cycle and cell-division events.
Which of the following best describes adaptor proteins?
- Adaptor proteins facilitate downstream signaling events but do not participate in signal transduction.
- Adaptor proteins help other proteins dock on phosphotyrosines so that they can be phosphorylated.
- Adaptor proteins organize the kinase cascade for ultimate efficiency.
- Adaptor proteins dimerize after a ligand binds to their extracellular membrane receptor.
- Adaptor proteins activate guanylate cyclase after a ligand binds to their extracellular membrane receptor.
Customer reviews
3,3 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
1 |
Hi, good day to everyone. I really wanted to make a review for this lecture, because for me it was a great brief and concise explanation. Thank you very much for the easygoing explanation Dr. Cornwall. I have a bachelor in cellular and molecular biochemistry from a US college and never seen anybody explain and organize all this material in a best possible simple way. English is my second language, and I had to study this topic both in English and in my original language, in collage, so I could be able to fully understand how they worked. This type of receptors and cascades involved, are a challenge to explain how they work in a very simple way; while you either try to understand by readding it on a textbook or see it on a picture, Dr. Cornwall does a really good job on how overall it works. To all my fellows studying this lecture, I just wanted to encorage you to remember that she is mainly explaining the overall function of the receptors, the enzymes and proteins involved, don't jump just yet to understanding all the types of pathways that go through the cascade. A quick tip for everyone to understand better this topic with this video: you could try to use both video and script at the same time, pause or rewind if you didn't capture what Dr Cornwall said, and re-read the script, review the slides with the images and write as you understand, that will make engage your brain and fully understand the topic as you go. Thank you again for your explanation Dr. Cornwall; thank you very much everyone for reading me and have a good study session. You all got this!
I dont know if its the diagram or what the doctor is saying but i just cant understand the MAP kinase and the GEF and everything else afterwards
complicated topic but I think I got it, though the Tyrosine kinase itself is a bit complex
Very disorganised way of explaining concepts It would be much better if things were presented in more detail and if the presentations were structured adequately