Playlist
Show Playlist
Hide Playlist
Proteomics (2d Gel Analysis) – Analytical Techniques in Biotechnology
-
13 Advanced BiotechnologyAnalyticalTechniques.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
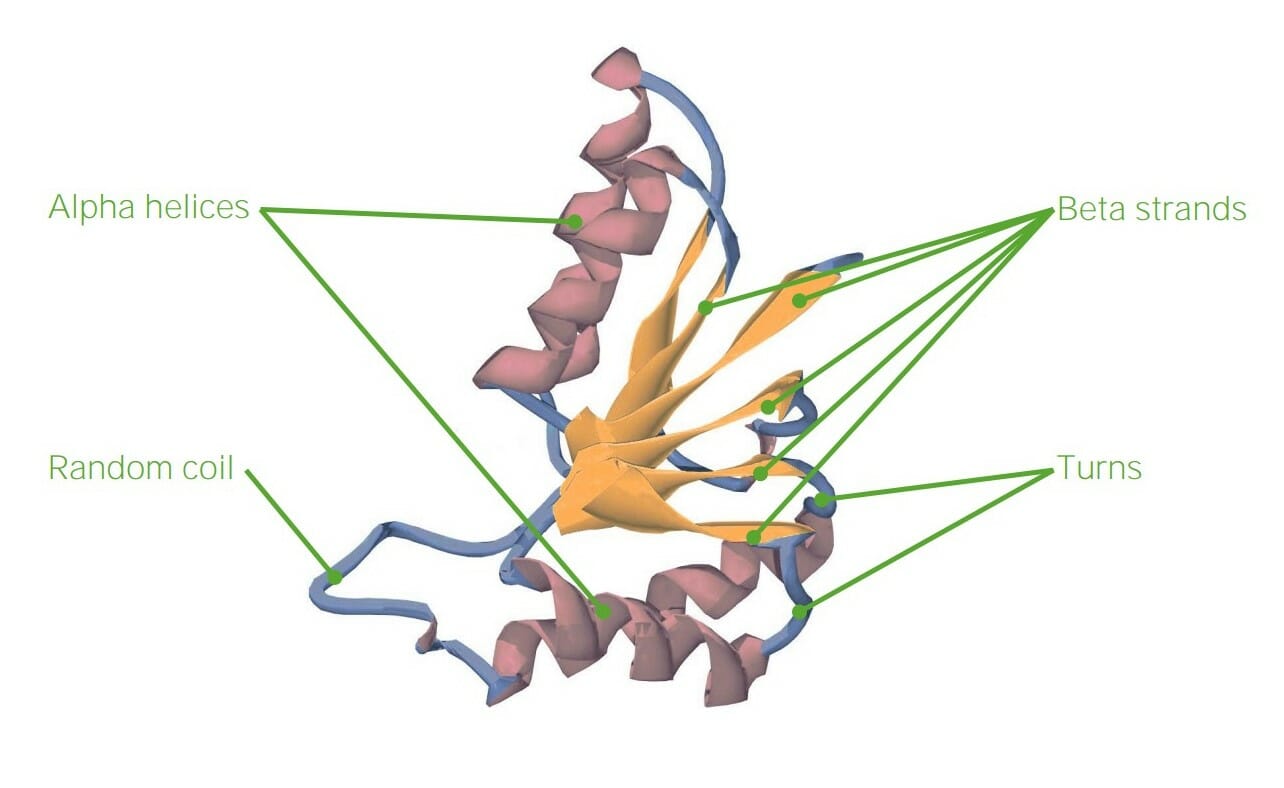
00:01 The last technique I wanna talk about here is that a proteomics. 00:05 So far we have talked about how to replicate DNA in incredible quantities. 00:08 We have talked about how to analyze the transcriptome or all the messenger RNAs of a cell. 00:15 Now we are gonna apply our technologies to understanding all of the proteins in a cell. 00:22 So in proteomics that's what we are after. We want to accomplish the same end proteomics that we had in transcriptomics. 00:30 We have to use some different techniques to accomplish that; because, of course proteins are different from RNAs. 00:37 One of the techniques that we used to do this is called 2D gel electrophoresis to accomplish it. So let me explain how that works. 00:45 There is two primary step process in this. 00:48 First, one takes a protein mixture that one has gotten from cells and applies it to what's called a polyelectrolyte column. Now this is a column that has material in it that has a variety of charges on it. 01:01 And charges, as you know, will segregate themselves according to the electrical field that you put them into. So if I have a tube of this material and I apply an electrical charge across it. 01:13 And let's say I put a positive at the top and a negative at the bottom then all of the ions that are in that column the negative ones that are the most negative are gonna travel the furthest to the top, close to the positive. 01:25 And the ones that are positive are gonna travel furthest down to the bottom closes to the negative, and between the two we will see a gradient. In the middle there be things that will have very little charge and then growing out to very large charges later. 01:41 Well what I have just done is that I have created a gradient of charge and that gradient of charge is very powerful for allowing me to separate proteins who have a variety of charges, okay? The proteins will separate according to their pI values, the place where the charge is 0. They will go to the place where charge is 0 and they will stop. 02:02 As so this gradient is a really powerful technique, the ones that are most positive and neutral pH are gonna be down below and the ones that are most negative are gonna be up at the top. 02:13 I'll take that gel. Now, this gel has already separated these proteins according to, basically, their charge. 02:19 It has separated them according to their charge and I lay this gel on top of another gel. 02:26 So, I have just done what we called the first dimension of the 2D gel, separated the proteins on the basis of their charge. 02:35 This column that I have just created is now applied to another gel that is going to separate them on the basis of their size. 02:44 So to do this I add a detergent called SDS. 02:48 And what SDS does is, it kinda neutralizes all the charges. So they don't effect the next analysis. 02:54 The next analysis is going to separate them only on the basis of their size not on their charge. 03:01 So we apply an electrical current from the top to the bottom and all of the proteins moved into the gel. 03:07 And the difference in this gel is the speed with which they move through it is a function of their size. 03:15 The smallest proteins will move the fastest. They travel the easiest route. 03:20 The largest proteins will be those that travel the slowest. 03:23 They have a more difficult time getting through the pores of the gel. 03:29 So the 2D are charged or pI have a role to think about that and the second dimension moving down in terms of size. 03:36 Well this is a result that you get if you do that kind of an analysis. 03:40 You see all the proteins of a cell now spread out into a 2D pattern. 03:46 The most positives and the largest, in this case, on the left. 03:52 The most negative and the largest on the right. 03:54 The neutral in the center and then by going up and down we can have the biggest and the smallest. 04:01 So for example I might know that a protein that I am interested in studying has a certain size. 04:05 It has a certain charge and I could find on this graph the spot of that protein corresponded to. 04:13 The beauty of this technique is, I can analyze every single protein; because, every spot on that gel corresponds to one protein. 04:22 And if I make my gel big enough I can get a spot for every single protein that's found in a cell. 04:29 Well, if you think about what we did in the last analysis where we compared the healthy cells versus the cancer cell, you can get an idea about what we can do with 2-D gel electrophoresis; because, let's think about that, if we start about with 2 sets of cells a healthy one and a cancerous one, then what we do is we label the proteins in one of them orange. 04:53 And we label the proteins in the other one, the cancer cell, blue. 04:57 When we run those on the gel, this is what we get. 05:02 So now we can see some differences corresponding to color like what we saw in the transcriptomics analysis. 05:09 Here are orange that are proteins that are in a healthy cell, but not in a cancer cell. 05:13 Things that are purely blue are proteins that are in a cancer cell but not in a healthy cell. 05:19 And proteins that are black are proteins that are equally abundant in both cells. 05:25 Now this kind of information can compliment the transcriptomics analysis very nicely to help us give a very precise piece of information about what cells have in them and then compare them between two different types of cells. 05:39 And this doesn't have to be a cancer cell. We could do the same kind of an analysis for either transcriptomics or for proteomics. Well let's say a drug. 05:47 May be we are interested in understanding the effect that a drug has on a healthy cell. 05:52 Where we take a health cell without a drug, take the same healthy cell with a drug and perform this analysis. 05:59 In many ways researches are limited only by their imagination in terms of the kinds of information they can ultimately get out. 06:06 Well, we have learned about three different techniques for analyzing the information in cells. 06:11 The information being the DNA, the RNA and the protein. 06:16 I hope that you have come away with the understanding that these techniques allows to determine things at a system level that would otherwise have been impossible 30 years ago.
About the Lecture
The lecture Proteomics (2d Gel Analysis) – Analytical Techniques in Biotechnology by Kevin Ahern, PhD is from the course Analytical Techniques in Biotechnology.
Included Quiz Questions
Which of the following is true of 2D gel electrophoresis?
- Each spot on the final gel corresponds to a single protein.
- The first step involves separating proteins by molecular weight.
- SDS is necessary to separate proteins by pI.
- The smallest proteins are found at the top of the gel.
- The largest proteins are separated towards the negative charge.
In the 2D-gel analysis, the first step involves the separation of proteins according to which of the following options?
- The charge or pI value
- The mass of the protein
- The molecular weight of the protein
- The size of the protein
- The shape of the protein
What is the function of sodium dodecyl sulfate in the second step of 2d-gel analysis?
- It helps in the neutralization of all the charges present on each protein molecule under investigation.
- It helps in protecting the negatively charged amino acids on the proteins from the attack of cations present in the electrophoretic buffer.
- It helps in protecting the positively charged amino acids on the proteins from the attack of anions present in the electrophoretic buffer.
- It provides a charge to the neutral proteins so that these can pass easily through the gel column during electrophoresis.
- It protects the proteins from degradation and activity loss stimulated under the effect of electric current.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
VERY clear explanation of how this works, I love Ahern's lectures.