Playlist
Show Playlist
Hide Playlist
Phases of Drug Development – Absorption and Distribution | Pharmacokinetics (PK)
-
Slides Phases Drug Development Absorption Distribution PK.pdf
-
Reference List Pharmacology.pdf
-
Download Lecture Overview
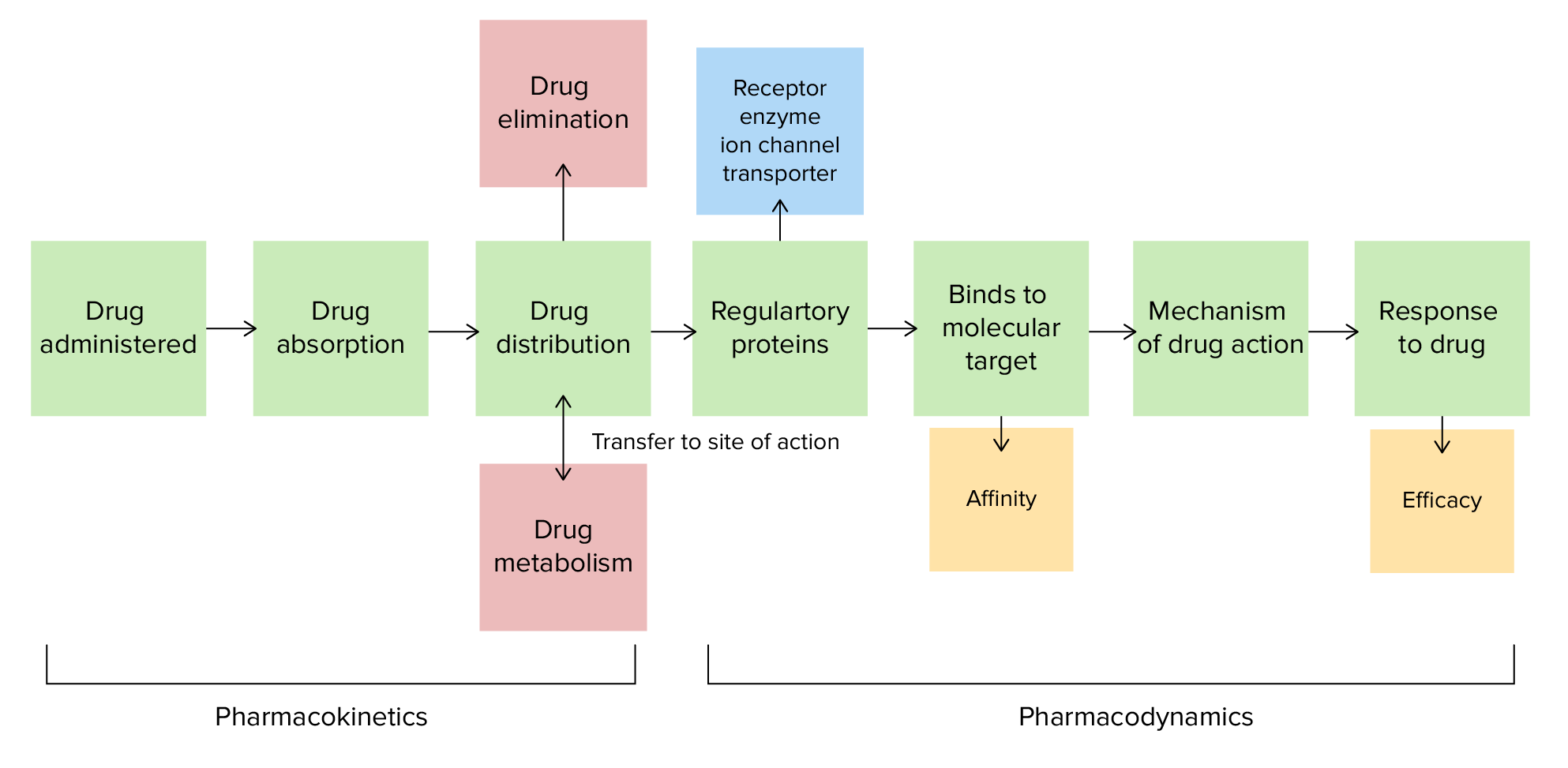
00:01 Okay, let's talk about something a little bit different. 00:04 Let's talk about phases of drug development. 00:06 So this mythical pharmaceutical company called Gargantua pharmaceuticals, has developed eyedrops that can give humans X-ray vision. 00:14 So far, rats have been able to see cheese through solid walls, and dogs can see chew toys through sheets of plywood. 00:22 Okay, sounds like a fun drug. 00:24 The chief executive officer comes to and wants to know, what is the next phase of drug development? Is it phase 1,2,3 OR 4? In order to answer this question, let's go through what the different phases are. 00:37 So when we're testing a drug, we talk about the preclinical phase. 00:41 This is where we do in vitro testing and little test tubes, and then little beakers. 00:45 And then we go on to animal testing, and then animal safety testing. 00:49 That's the preclinical phase. 00:51 Then we move on to the clinical phase, which is in humans, phase 1,2,3, and 4. 00:56 When we talk about in vitro testing, we're really talking about various chemical parameters. 01:02 We measure pKa, we measure tissue stability, tissue binding, whether or not a drug is sensitive to light or not, whether or not it'll actually leach into the plastic of a pill container. 01:14 All kinds of things are tested at this stage of development. 01:18 We do environmental testing, because we want to know if this drug is dropped into the sewer, is it going to cause damage to fish and to wildlife. 01:28 And we do stability testing, whether it's hot temperatures, if you leave the drug on the dash of your car, or if you accidentally let it freeze in a cold climate. 01:39 Animal testing is always tested on at least two species. 01:43 we test acute toxicity and subacute toxicity. 01:48 And then we do animal safety testing, which is a pharmacologic profile and reproductive toxicity. 01:54 So let's move on to the clinical phase. 01:56 The clinical phase can be phase 1, 2, 3 or 4. 02:00 In phase 1 studies, we do dose response relationships in healthy male volunteers. 02:06 In the United States, we can do anywhere from 20 to 200 volunteers. 02:10 In the United Kingdom, and in Canada, it's generally between 80 and 200 volunteers. 02:15 You may use volunteers in smaller numbers in cancer patients as well. 02:22 In terms of evaluation of patients with disease, now in phase two studies, they are much larger 100 to 250 patients, sometimes they're even larger than that. 02:31 There's usually with a placebo controlled arm, and pharmacokinetic and pharmacodynamic data is collected at this point. 02:39 Once this information is definitively analyzed and definitively verified, independently, we move on to phase 3 trials. 02:49 So phase3 trials are the big ones that we always talk about. 02:53 There's generally a placebo arm. 02:56 Generally, it's compared against oral therapy or old, pardon me, older therapy. 03:02 We monitor for rare forms of toxicities and adverse events. 03:07 And if that phase 3 study is successful, the drug company will submit a new drug application or NDA, to the FDA. 03:16 In Canada, that NDA goes to the Health Protection Branch. 03:20 And in the European Union, there are multiple agencies like the EMA or CHMP, or sometimes national organizations or subnational organizations that will be able to evaluate this for them. 03:33 Phase 4 refers to post marketing surveillance. 03:37 Manufacturers are always required globally to inform the FDA at regular intervals, and inform the regulatory agency within whatever country that they're operating in. 03:47 It depends upon you. 03:48 So you as a physician need to notify the drug company if you see an adverse event. 03:54 I personally have filled out probably 2 or 3000 phase 4 forms in over the course of my career, So it's an important part of phase, it's an important part in the development of drugs, and you play a very important part. 04:07 So let's go back to our question. 04:09 We were asking whether or not the next phase of development is phase 1,2,3 or 4 . 04:15 Well, clearly Gargantua pharmaceuticals finished or completed much of their animal trials, they're ready for human trials. 04:22 So the answer is going to be phase one. 04:25 And in case you're wondering, yes, that's my dog Tucker in the picture. 04:28 And he always lies like that each morning and watches me get dressed. 04:32 I hope you enjoyed this lecture. 04:33 And if you have any questions, you can certainly email us. 04:36 There are more lectures to follow. 04:38 And we're going to ask lots of questions and we're going to make you much better at writing your exam.
About the Lecture
The lecture Phases of Drug Development – Absorption and Distribution | Pharmacokinetics (PK) by Pravin Shukle, MD is from the course Pharmacokinetics and Pharmacodynamics.
Included Quiz Questions
Which clinical trial phase is a randomized controlled multicenter trial from a large patient group aimed at establishing the effectiveness of a drug?
- Phase 3
- Phase 1
- Phase 2
- Phase 4
- Phase 0
What is NOT performed in the preclinical phase of drug testing?
- Testing on healthy human volunteers
- Environmental testing
- In vitro testing
- In vivo testing
- Testing on human tissues
Which clinical trial phase primarily deals with dose-response in healthy volunteers?
- Phase 1
- Phase 2
- Phase 3
- Phase 4
- Phase 0
Post-marketing surveillance of a new drug is performed during which clinical trial phase?
- Phase 4
- Phase 3
- Phase 0
- Phase 1
- Phase 2
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Very clean and understandable lecture with charts and perfect voice of the lecture