Playlist
Show Playlist
Hide Playlist
Oxidation of Unsaturated and Other Fatty Acids
-
07 Advanced LipidMetabolism-Fat&FattyAcids.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
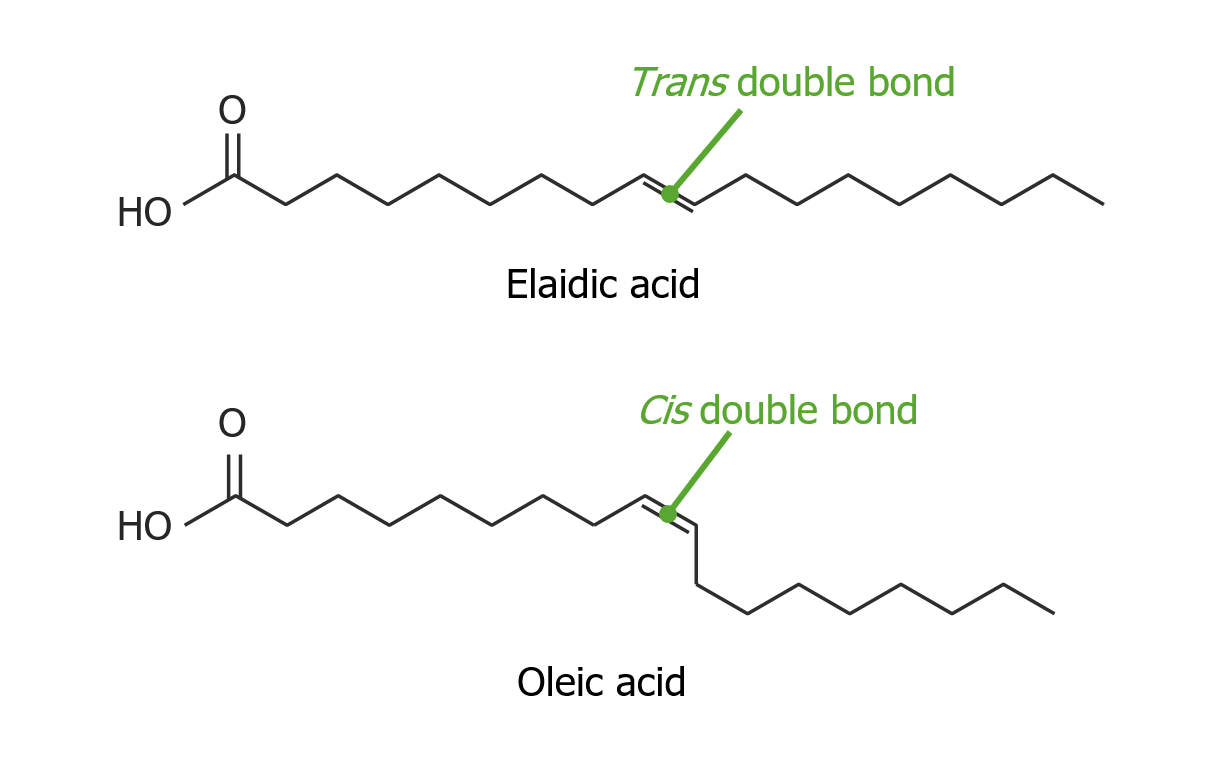
00:01 Now the oxidation of unsaturated fatty acids posses a little bit of a problem for a cell; because, if you recall the unsaturated fatty acids have cis-double bonds whereas the beta oxidation, I have just described to you, creates intermediates that have trans double bonds. 00:16 So those have to be dealt with appropriately. 00:18 I show on the screen the way in which this is handled and it's handled interesting by only two enzymes that it requires to do it. 00:25 So I show the fatty acids that has 2 double bonds on the top and the oxidation of that fatty acid proceeds by normal beta oxidation until the oxidation process gets close to that cis-double bond. 00:38 When that happens we create an intermediate that looks here like this one that has a double at positions 3 and 4. 00:45 Now you remember in the beta oxidation that the trans double bond was at positions 2 and 3 whereas we have a cis at positions 3 and 4 in the structure. Now the cis 3,4 double bond in this is handled very easily by an enzyme called Enoyl-CoA isomerase. 01:02 The cis 3,4 double bond is converted to a trans 2,3 double bond. 01:05 Now that trans 2,3 double bond is an intermediate normally in beta oxidation, we can see that happening right here. 01:14 That bond is then metabolized and broken down and two carbons are broken off just like it has happened before in beta oxidation. 01:22 After that two carbon pieces being released we start the process again of oxidizing what remains and we get to an intermediate that has a trans 2,3 bond next to a cis 4,5. Now this is getting a little tacky but the important thing is that the cell can't handle those. 01:42 So rather than deal, we tried to deal with those two use another enzyme that comes into play that's start to simplify that situation. 01:48 And that enzyme is shown here, it's 2,4 Dienoyl-CoA reductase. 01:54 And it uses electrons, because, it has to do a reduction at this point in order to convert two double bonds into one double bond and that's what this enzyme is doing. 02:04 So it's converting the two double bonds that you see here into one double bond and the one double bond is between carbons 3 and 4. 02:14 Well, the one double bond between carbons 3 and 4 is converted into a double bond between carbons 2 and 3 by the same enzyme we saw before, Enoyl-CoA isomerase. 02:24 At this point we have an intermediate that has a trans bond in positions 2 and 3 and beta oxidation can continue as normal. 02:32 Now long chain fatty acids are metabolized slightly differently than shorter chain fatty acids and by long chain, I typically mean fatty acids that are 20-22 carbons or greater. 02:45 The oxidations of these starts not in the mitochondrion but in another organelle called the peroxisome. 02:52 And fatty acids with odd numbers of carbons which the cell occasionally encounters are oxidized in a little bit different process; because, most fatty acids have even numbers of carbons and even numbers of carbons work really well. 03:05 Because if you are chopping off two carbon pieces every time when you get to the last 4 carbon pieces you split it in half and you have two units of 2 carbons each acetyl-CoA. 03:16 Now if you have an odd number of carbons in your fatty acid chain then that means when you get down to the end, and instead of having a 4 carbon piece you have a 3 carbon piece and that 3 carbon piece is called propionyl-CoA. 03:28 Propionyl-CoA has to be altered in some way for the cell to be able to handle it and it's handled in this way by a certain unusual scheme. 03:38 First there is an enzyme called propionyl-CoA carboxylase which combines with bicarbonate to add a carboxyl group to the propionyl-CoA creating methylmalonyl-CoA that you can see right here. 03:52 Now you will think move forward from there but the cell doesn't move from there. It actually converts the forms and it converts the forms from the D to the L configuration before going forwards. As it goes forwards then it takes that carboxyl group that it added to the end and rearranges it so that it creates a new molecule called auccinyl-CoA. 04:15 That enzyme that catalyzes this reaction is called methylmalonyl-CoA mutase, mouth full of a name. 04:22 But this enzyme requires cobalt and this enzyme requires vitamin B12. 04:28 So, it's one of the reasons that we have to have B12 in our diet; because, this reaction very much depends on it. 04:34 Succinyl-CoA is an intermediate in the citric acid cycle and can be metabolized there. 04:42 Now each round of fatty acid oxidation creates one molecule of FADH2 and one NADH, as we have seen. 04:47 Along with one acetyl-CoA and a fatty acid is been shorten by two carbons. 04:52 Each acetyl-CoA that's released in the matrix of the mitochondrion and this is important; because, this is where the citric acid cycle actually uses those. So it doesn't have to go anywhere else.
About the Lecture
The lecture Oxidation of Unsaturated and Other Fatty Acids by Kevin Ahern, PhD is from the course Lipid Metabolism.
Included Quiz Questions
What occurs in the first step of fatty acid oxidation?
- Protons and electrons are removed.
- Water is added.
- NADH is produced.
- A cis-intermediate is created.
What occurs when water is added during fatty acid oxidation?
- A double bond is destroyed.
- An intermediate in the D configuration is created.
- A ketone is created.
- A trans intermediate is created.
In the reaction of fatty acid oxidation that makes NADH, which of the following is produced?
- A ketone.
- Alcohol
- A cis-double bond.
- A trans-double bond.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Very eloquent :) my favorite on this website! would definitely recommend.