Playlist
Show Playlist
Hide Playlist
Overview and Introduction on Fatty Acids – Lipids
-
08 Basic Lipids-Fats&Oils.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
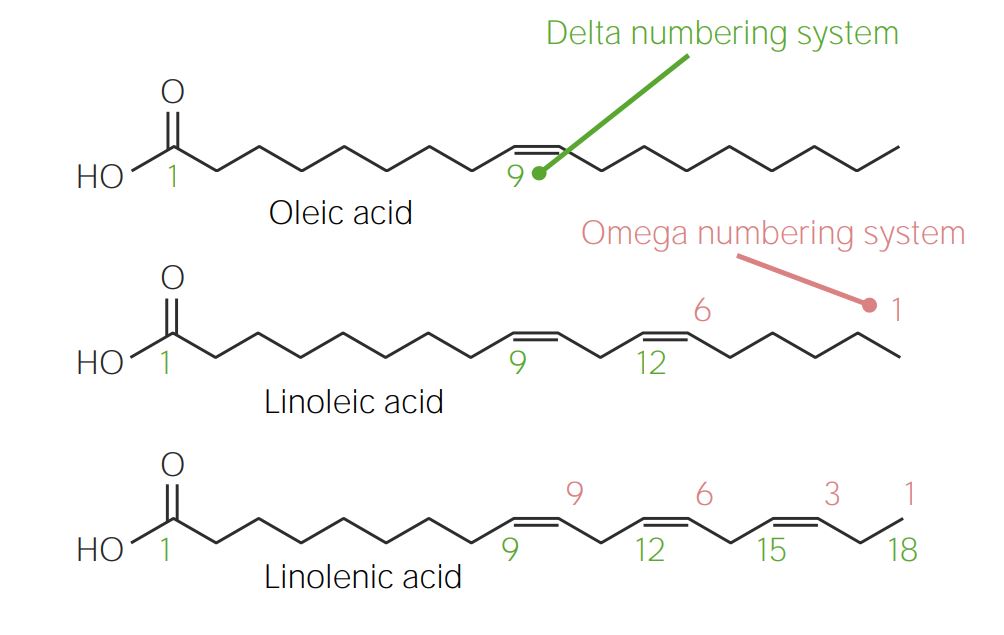
00:01 Das Wort "Fett" mag vielen Menschen Angst einjagen, denn es stimmt, dass Fette bei Tieren und Öle bei Pflanzen wichtige Energiespeicher sind. 00:11 Auf dem Bild rechts sehen Sie eine fettleibige Maus und eine normale Maus. 00:15 Der fettleibigen Maus fehlt ein Gen namens Leptin, was ihre Fettleibigkeit ausgelöst hat. 00:20 Lipide sind Verbindungen, die die Kategorie der Fette und Öle umfassen, und in diesem Vortrag werde ich nun über einige dieser Komponenten sprechen. 00:28 Dazu gehören Fettsäuren, Glycerolipide, Sphingolipide und Polyketide. 00:34 Fettsäuren sind ein Teil dessen, was ein Fettmolekül ausmacht und Fettsäuren sind insofern interessante Lipide, als dass sie amphiphil sind. 00:43 Amphiphile Moleküle sind Moleküle, die einen Teil haben, der sehr polar ist und mit Wasser interagieren kann und ein anderer Teil ist unpolar und kann nicht mit Wasser interagieren. 00:55 Fettsäuren werden natürlich zur Herstellung von Seifen verwendet und variieren in ihrer Sättigung. 01:02 Die Sättigung bezieht sich auf die Anzahl der Einfachbindungen, die sie haben. 01:06 Je weniger gesättigt ein Fett ist, desto mehr Doppelbindungen enthält es. 01:10 Fette oder Fettsäuren variieren auch in ihrer Länge. 01:15 Hier sehen wir die häufigsten Fettsäuren, die in Zellen vorkommen. 01:20 Dies sind die gesättigten Fettsäuren, und Sie können feststellen, dass sie sich in ihrer Größe um jeweils zwei Kohlenstoffe unterscheiden. 01:26 Die kleinste ist die Laurinsäure mit 12 und die Myristinsäure mit 14 Kohlenstoffen, Palmitinsäure mit 16, Stearinsäure mit 18 und Arachidonsäure mit 20 Kohlenstoffen. 01:36 Der Grund dafür, dass sie sich in der Anzahl der Kohlenstoffe um zwei unterscheiden, ist, dass die Synthese von Fetten mit einer Einheit erfolgt, die jedes Mal zwei Kohlenstoffe hinzufügt. 01:45 Dies hier ist eine Darstellung der Struktur einer der Fettsäuren, der Stearinsäure. 01:50 Und auf der linken Seite sehen Sie eine Carboxylgruppe, die bei physiologischem pH-Wert ionisieren kann. 01:57 Der Rest des Moleküls enthält nur Kohlenstoff und Wasserstoff und ist sehr unpolar. 02:01 Aus diesem Grund bezeichnen wir Fettsäuren als amphiphil. 02:06 Fettsäuren sind ebenfalls ungesättigt. 02:10 Das heißt, einige Fettsäuren enthalten nicht alle Einfachbindungen. 02:14 Palmölsäure zum Beispiel enthält 16 Kohlenstoffe, aber eine der Bindungen in diesem Molekül ist eine Doppelbindung. 02:21 Ölsäure enthält 18 Kohlenstoffe mit einer Doppelbindung, und Sie können sehen, dass Linolsäure 18 mit zwei Doppelbindungen hat, Linolensäure 18 mit drei Doppelbindungen, und Arachidonsäure hat 20 Kohlenstoffe mit vier Doppelbindungen. 02:33 Jede dieser Fettsäuren ist sehr wichtig für die Herstellung von Fetten und den Komponenten, aus denen unsere Membranen bestehen. 02:41 Hier sehen Sie die Struktur der Ölsäure, und Sie können die Doppelbindung in der Ölsäure erkennen. 02:45 Die biologischen Fettsäuren enthalten fast immer eine Doppelbindung in der cis-Konfiguration. 02:51 Die meisten Menschen haben von Transfetten gehört, und Transfette entstehen in der Regel durch die chemische Veränderung von Lebensmitteln und sind mit einem gewissen Gesundheitsrisiko verbunden. 03:04 Sie können hier auf dem Bildschirm eine häufige Transfettsäure, die Elaidinsäure, sehen. 03:09 Als Vergleich haben wir hier Ölsäure und Sie können den Unterschied in der Form dieser Moleküle erkennen. 03:13 Die cis-Bindung ist in normalen biologischen - biologisch produzierten Fettsäuren enthalten und hat eine physische Biegung in ihrem Inneren.
About the Lecture
The lecture Overview and Introduction on Fatty Acids – Lipids by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which is true of unsaturated fatty acids?
- They are usually in the cis configuration in cells
- They include stearic and palmitic acid
- They increase the Tm of the membranes they are found in
- They are more common in the membranes of people than those of fish
- They do not contain double bonds.
Which of the following is a trans fatty acid?
- Elaidic acid
- Arachidonic acid
- Linoleic acid
- Linolenic acid
- Oleic acid
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Very nice and informative lecture, easy to understand and remember