Playlist
Show Playlist
Hide Playlist
Movement of CO₂
-
Slides Hemoglobin Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
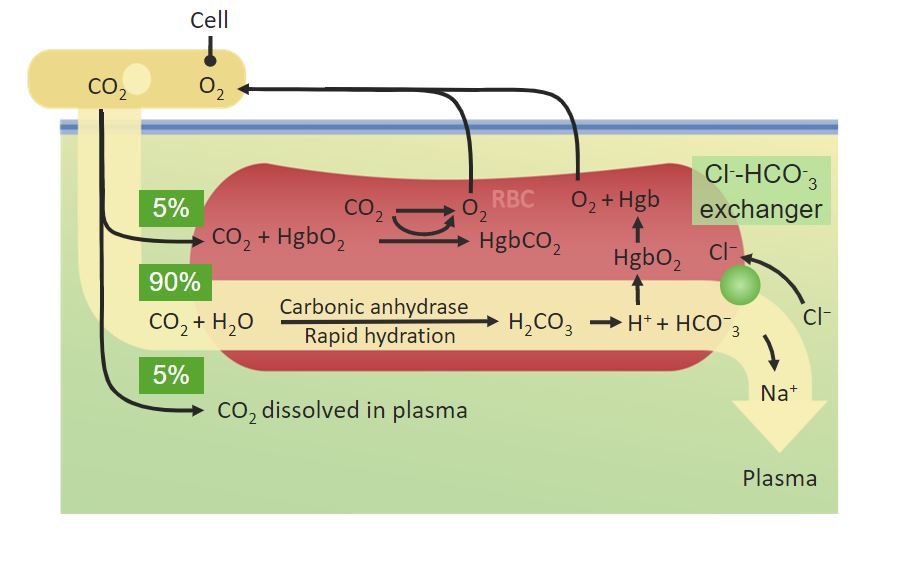
00:02 So, we've seen hemoglobin does some really remarkable things. 00:05 One of the things that I have not talked about is how hemoglobin is affected by carbon dioxide, and that's what I want to spend just a little bit of time talking about here. 00:14 Carbon dioxide is moved in the body by a couple of processes, one of which involves hemoglobin. 00:20 Now, I want to emphasize first of all, that carbon monoxide can bind to the heme unit and compete with oxygen because it has a shape very much like an oxygen molecule. 00:29 Carbon dioxide cannot do that. 00:31 It binds to a different portion of hemoglobin. 00:34 So what I'm talking about here is not competing with oxygen, but rather binding to another part of the hemoglobin molecule. 00:41 That binding the other part of the hemoglobin also causes some changes in hemoglobin which is why the hemoglobin releases the oxygen molecule. 00:49 Well, let's look at the path that a carbon dioxide molecule takes in going from a rapidly metabolizing tissue to get back to the lungs so that it can be exhaled. 00:59 We see carbon dioxide at the very top and we see the carbon dioxide can combine with hemoglobin. 01:06 And when this happens in the presence of protons, oxygen is released and the carbon dioxide is bound to the hemoglobin as you can see here. 01:15 That only accounts for a relatively small portion of the carbon dioxide that needs to travel back to the lungs. 01:22 The remainder of the carbon dioxide gets solubilized and it gets solubilized by an enzyme that converts carbon dioxide into carbonic acid as you can see here. 01:34 The enzyme is known as carbonic anhydrase and it's a very, very efficient enzyme producing carbonic acid the bottom molecule shown right here. 01:43 Well, carbonic acid is an acid and at the pH of the blood readily loses its proton. 01:50 When that happens, it becomes the ion at the bottom known as bicarbonate. 01:54 So bicarbonate is a traveling form of carbon dioxide solubilized in the blood. 02:02 A longer travel back to the lungs, the blood, if it's still carrying oxygen, as you can see here, can exchange that oxygen for carbon dioxide through the carbonic acid reversal pathway to give additional CO2 and allow the hemoglobin to pick it up as we can see here. 02:19 When we get back to the lungs, this hemoglobin that's bound to carbon dioxide, on the other hand, has a very different situation that existed inside of the body. 02:28 Remember in the lungs that the oxygen concentration is very high and oxygen forces its way on to the hemoglobin that's bound to that carbon dioxide. 02:38 The hemoglobin, as you can see here, gains an oxygen. 02:41 And in the process, loses the carbon dioxide that it had. 02:45 So we see the oxygen coming on. 02:47 We see the water and we see the carbon dioxide being released here. 02:51 And we also see the carbonic acid, which is in the form of bicarbonate, being converted into oxygen and carbon dioxide. 02:59 All of these help favor the exhaling of CO2. 03:02 So hemoglobin carrying carbon dioxide and bicarbonate dissolved in the blood, both come together at the very end as they reach the lungs and cause carbon dioxide to be exhaled. 03:15 That's critical because carbon dioxide in high concentrations will kill you. 03:20 As I mentioned earlier, carbon monoxide competes with oxygen for binding to the iron atom of the heme group. 03:26 Now, this is important because obviously we want to be carrying oxygen not carbon monoxide in our hemoglobin. 03:33 And it turns out that there's yet another histidine that's present in the environment of the iron as you can see in the figure here. 03:42 In addition to having histidine below the heme, which is physically attached to the iron atom, there's another histidine above the heme group that is not attached to the iron. 03:53 This above histidine causes the carbon monoxide to actually have a lesser affinity for the heme group. 04:01 It doesn’t affect oxygen in the same way that it affects the carbon monoxide. 04:05 Now, this means that because of the presence of this histidine atom above the iron atom that the oxygen is much more likely to bind than carbon monoxide is. 04:16 High enough concentrations carbon monoxide is going to be a problem, but not as much as it would be if that histidine wasn't there. 04:24 So, even though carbon monoxide is present and it's present in the lungs of smokers, for example, it doesn’t reach toxic enough concentrations unless you get a higher concentration that is present in cigarette smoke. 04:37 Nonetheless, you don’t want to have anymore carbon monoxide than necessary.
About the Lecture
The lecture Movement of CO₂ by Kevin Ahern, PhD is from the course Amino Acid Metabolism.
Included Quiz Questions
Which enzyme helps in the conversion of CO2 to carbonic acid during its transportation from tissues to the lungs?
- Carbonic anhydrase
- Carbonic hydrase
- Carbonic decarboxylase
- Carbonic lyase
- Carbonic ligase
Which of the following statements is not true?
- Carbon dioxide competes with oxygen to bind to the heme unit of the hemoglobin.
- The binding of CO2 to the hemoglobin leads to a structural change in the hemoglobin and causes the release of O2 from the heme group.
- The majority of transported CO2, to the lungs, is in the form of bicarbonate.
- Carbon monoxide competes with oxygen to bind with the Fe atom of the heme group in hemoglobin.
- A histidine entity present above the heme group in the hemoglobin favors the binding of oxygen over CO.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |