Playlist
Show Playlist
Hide Playlist
Michaelis-Menten Kinetics: Considerations – Enzyme Classification
-
02 Advanced Enzymes&Kinetics2.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
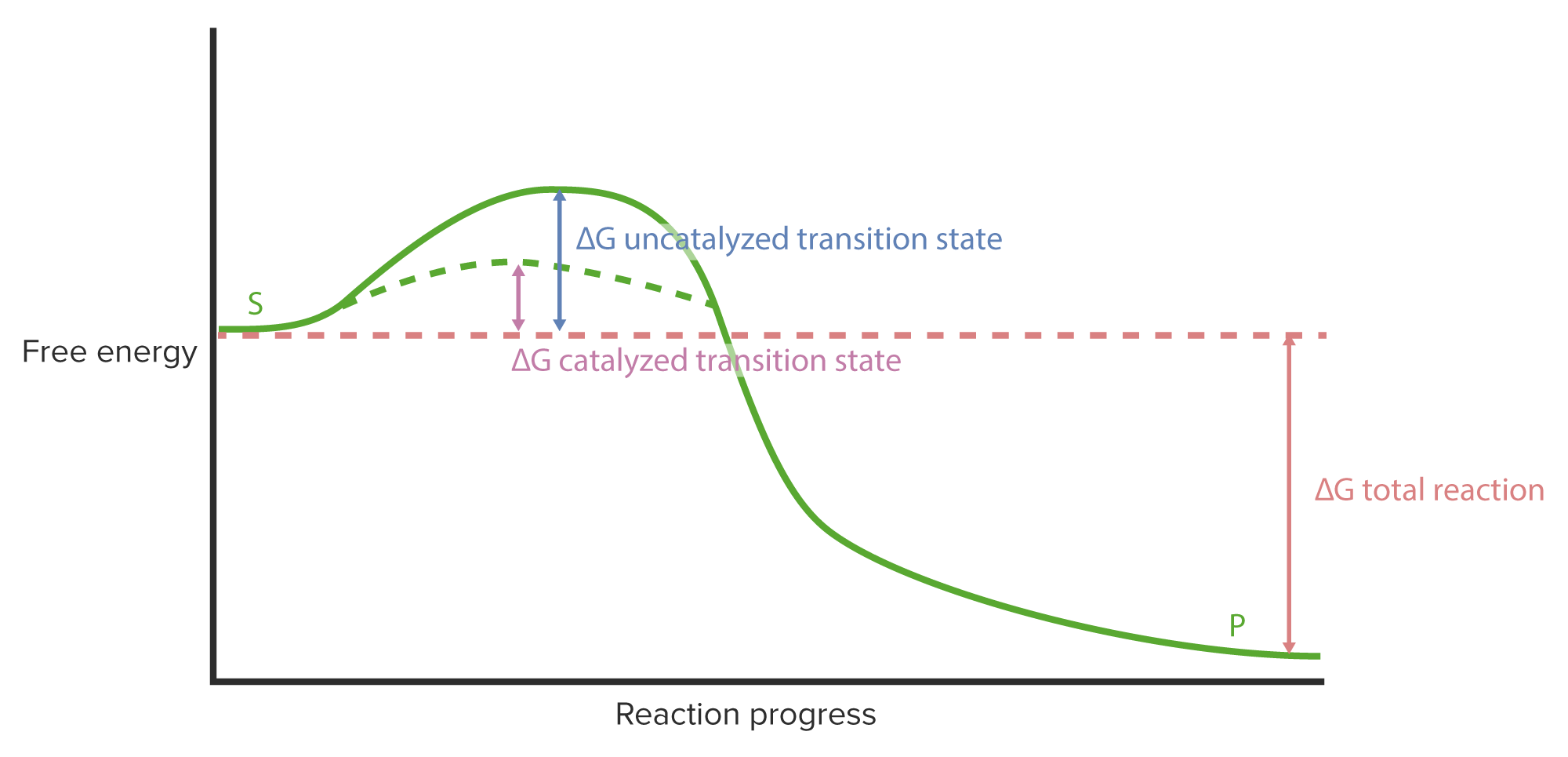
00:01 Die Geschwindigkeiten enzymatischer Reaktion und die Affinität von Enzymen zu ihren Substraten sind wichtige Konzepte zum Verständnis. 00:07 Diesen Themen werde ich mich im ersten Teil dieses Vortrags zur Datenanalyse widmen. 00:12 Ich werde auch den Allosterismus und seine Auswirkungen auf Enzyme und ihre Katalyse behandeln. 00:18 Danach werde ich über die Substratbindung sprechen und mit welchen verschiedenen Mechanismen dies innerhalb von Enzymen geschehen kann. 00:23 Als letztes werde ich über die Weise sprechen, wie wir Enzyme klassifizieren und Reaktionen in einem sehr weiten Sinne verstehen. 00:31 Okay, unter Betrachtung der Michaelis-Menten-Kinetik haben wir gelernt, dass es für uns wichtig ist, enzymatische Reaktionen unter Steady-State-Bedingungen zu beobachten. Das heißt: zu dem Zeitpunkt, an dem relativ konstante Mengen des ES-Komplexes vorliegen. 00:44 Unter der Michaelis-Menten-Kinetik gilt die Gleichung dort oben und diese Gleichung sagt uns einige wichtige Dinge, die wir in dieser Vorlesung lernen werden. 00:52 V0 - also die Reaktionsgeschwindigkeit - ist gleich Vmax - das werden wir gleich besprechen - mal die Substratkonzentration, geteilt durch eine andere Größe namens Km - die wir auch besprechen werden - plus die Substratkonzentration. 01:05 Wir haben hier also zwei Begriffe gelernt, die wichtig für unser Verständnis sind, und zwar Vmax - die maximale Reaktionsgeschwindigkeit - und Km - eine Größe, mit der wir die Affinität eines Enzyms für sein Substrat messen können. 01:20 Beginnen wir zunächst mit Vmax. Bei Vmax ist es wichtig zu verstehen, was es ist, warum es so ist und wie es passiert. 01:28 Als wir V0 gegen die Substratkonzentration aufgetragen haben, haben wir gesehen, dass die Kurve anstieg und sich dann abflachte. 01:38 Und der Grund dafür, dass sie sich abflacht, liegt in der Art und Weise, wie Enzyme arbeiten und wie sie mit Substraten interagieren. 01:44 Anstelle dass die enzymatische Reaktion bei steigenden Substratkonzentrationen linear verläuft, passiert folgendes: Enzyme werden mit Substrat gesättigt. 01:55 Substratsättigung bedeutet, dass das Enzym fast ständig Substrat bindet, das heißt, wir haben fast alles im ES-Komplex. 02:06 Also bei sehr hohen Substratkonzentrationen setzt das Enzym kontinuierlich Produkt frei. 02:15 Und im Laufe der Zeit, wenn wir mehr und mehr Substrat hinzufügen, überschreiten wir die Kapazität des Enzyms, mehr Substrat zu binden. 02:21 Also unter gesättigten Substratbedingungen, bleibt das Enzym nicht mehr linear und es flacht ab. Wir sehen also diese Hyperbel. 02:32 Ein Beispiel könnte eine Fabrik sein, die Produkte herstellt. Eine Fabrik, die Produkte herstellt, wird eine Menge Arbeiter haben. 02:38 Und diese Arbeiter arbeiten an etwas, aber wenn sie nicht genug Material haben, um ein Produkt herzustellen, dann werden diese Arbeiter eine ganze Weile herumstehen und warten, bis sie wieder ein Produkt herstellen können. 02:49 Andererseits könnten wir uns vorstellen, dass, wenn wir dieselben Arbeiter haben und auch alle Materialien, die sie zur Herstellung von Produkten benötigen, sie eine bestimmte Anzahl von Produkten pro Tag herstellen werden. 03:01 Wenn wir die Menge der Materialien erhöhen, aber nicht die Anzahl der Arbeiter, verändern wir nicht das Maximum, das sie verarbeiten können. Das Gleiche passiert in der realen Welt bei enzymatischen Reaktionen. 03:14 Wenn wir die Menge des Produkts erhöhen wollen, müssen wir mehr Arbeiter einstellen, vielleicht eine weitere Fabrik bauen um mehr Produkte herstellen zu können. 03:23 Nun, es gibt auch Enzyme, die nicht der Michaelis-Menten-Kinetik unterliegen, zum Beispiel solche, die Substrate kooperativ binden. 03:30 In einer anderen Präsentation habe ich darüber gesprochen, wie Hämoglobin kooperativ an Sauerstoff bindet. 03:36 Und das bedeutet, dass die Bindung eines Substrats die Bindung eines Anderen beeinflusst. 03:42 Wenn das passiert - und natürlich passiert das nur bei Proteinen mit mehreren Untereinheiten - wenn dies geschieht, wenn die Bindung des Einen die des Anderen beeinflusst, dann werden wir natürlich eine Änderung der Reaktionsgeschwindigkeit beobachten; denn dies verändert die Bindungsvoraussetzungen des Enzyms. 03:57 Wenn diese Dinge passieren, können wir sie ziemlich leicht erkennen; denn dann bekommen wir eine "S"-Kurve im V/[S]-Diagramm, sehr ähnlich zu dem, was wir bei der Bindung von Hämoglobin an Sauerstoff gesehen haben.
About the Lecture
The lecture Michaelis-Menten Kinetics: Considerations – Enzyme Classification by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
What is the shape of the curve describing the relationship between substrate concentration and reaction rate, following Michaelis-Menten kinetics?
- Hyperbolic
- Linear
- Parabolic
- Sigmoidal
- U-shaped
The Km constant in Michaelis Menten kinetics refers to which of the following?
- The affinity of the enzyme for its substrate.
- The affinity of the enzyme for the final product.
- The velocity of the reaction, at a steady-state.
- The rate of reaction at the initial phase of reaction at higher substrate concentrations.
- The rate of reverse reaction.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
3 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Perfect way of explaining complex things. This is rarely seen by lecturers. Wow!
Professor Ahern is a perfect teacher and he makes everything so easy to understand
I understood perfectly the concepts the way he explained them. He makes it ease to grasp