Playlist
Show Playlist
Hide Playlist
Measuring Ions and Fluid Compartments
-
Slides 08 FluidCompartments UrinarySystem.pdf
-
Download Lecture Overview
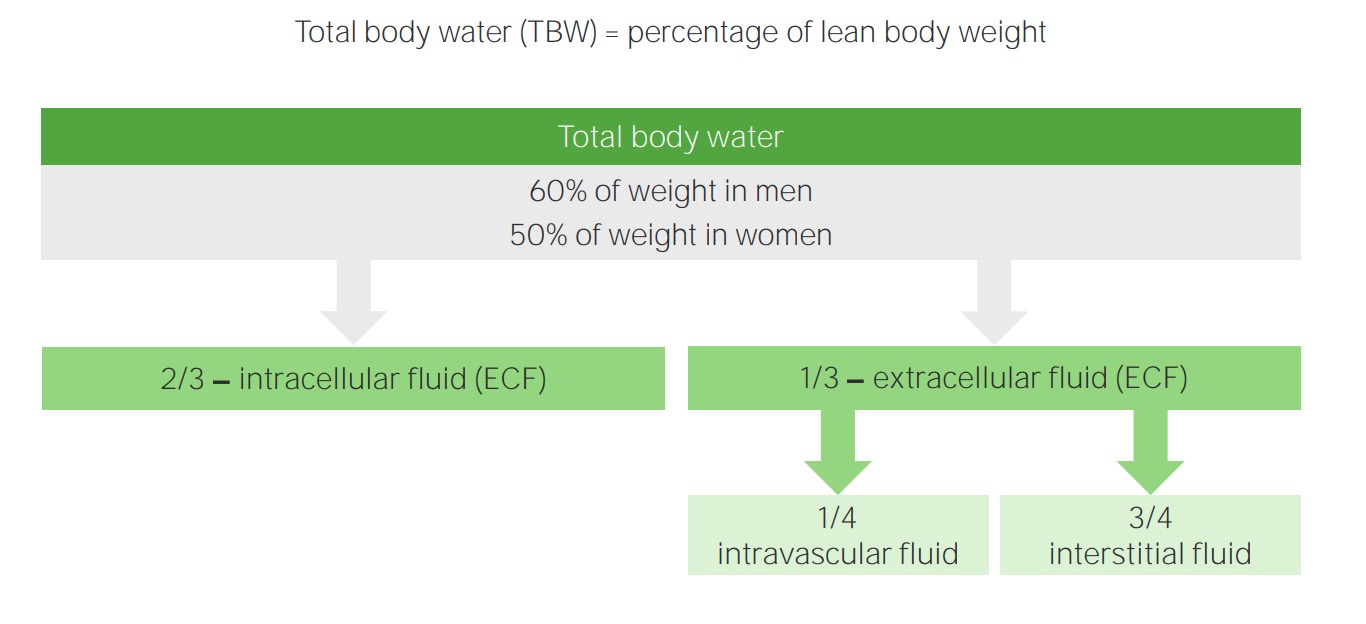
00:01 Now we're going to take a look at how you measure the ions and fluid compartments. 00:06 In the US, we oftentimes use something like a basic metabolic panel, This involves eight different substances, sodium, potassium, chloride, bicarb, calcium, glucose, blood urea nitrogen, and creatinine. 00:24 If you want to get a little bit more expanded panel, you can get other things like the total protein concentration, and albumin. 00:32 These are all very important aspects to get a good feel for what are the ions, the osmolality potential, as well as the osmotic potential. 00:43 If you want to measure osmolality directly, you have to put it into a machine. 00:48 We do that with either a freeze point depression or a vapor depression. 00:52 If you don't have those available and only have the chem-7, you can calculate it. 00:58 And this calculation is just done by this formula. 01:01 You take two times the sodium concentration, glucose divided by 18, and the blood urea nitrogen divided by 2.8. 01:09 So I provide you just an example here at the bottom. 01:12 These are very typical sodium values of a 140 glucose value of 80 milligrams per deciliter, and a BUN of 8 milligrams per deciliter. 01:23 If you go through the formula, it comes out to be 287 milliosmoles. 01:28 And this is a very typical blood value. 01:31 So the interstitium and the blood will be a right around this 285 to 287. 01:37 The intracellular component isn't just a teeny bit higher, maybe around 300 milliosmoles. 01:42 So this is what is going to be circulating around in the body, in comparison now to what is within a cell. 01:49 We don't really know what cell osmolality is on a minute to minute basis, because we don't really puncture the cell to measure its fluid concentration. 01:59 We only measure what's in the blood. 02:01 And that is why measuring blood levels of various substances is very important for us clinically. 02:09 Now, what else is important besides the osmolality, and that is the oncotic pressure. 02:16 So oncotic pressures help us to determine if we're going to move fluid into a blood vessel, or it's going to be moved out of the blood vessel. 02:24 And this is based upon proteins. 02:26 So we have the total protein concentration, the albumin, which is the major component of a serum protein analysis. 02:36 We have a few globulins that are present, but these are more minor in nature. 02:40 So we lose the look at total protein and albumin as our primary factors to determine the oncotic pressure. 02:47 Another important measurement for body fluid balance is the hematocrit. 02:53 When a person measures the hematocrit, they basically take whole blood. 02:58 You spin it down in a centrifuge and it breaks out into three layers. 03:03 The first layer is the plasma component. 03:05 And that is where of course, we found the plasma proteins that we just discussed. 03:10 There's also a Buffy layer. 03:12 And this Buffy coat is white blood cells and platelets. 03:18 There is also a red blood cell component, and that is what makes up the hematocrit. 03:23 So if you take the percent of the red blood cells, or the red blood cell component, divided by the total, you will get a percent number. 03:32 Now how do we use this particular percent number in body fluid balance? The most important thing to think about here is that we still have our same plasma component, our Buffy coat, and our hematocrit. 03:48 Her hematocrit, though, is really packed red blood cells. 03:52 So if you think about there's a certain number of red blood cells present. 03:57 If you have cell swelling occurring of the red blood cells, such as if you put the blood into a hypo-osmotic solution, they will increase their size. 04:12 If they increase their size, even though the number of red blood cells haven't changed, it will accomplish more of the total and therefore have a higher hematocrit. 04:25 The same number of red blood cells, if in a hyperosmotic solution will shrink. 04:33 And if they shrink, even though there's a same number of red blood cells, they will have less height than the total height and therefore it will look like there's a decrease in the hematocrit. 04:46 So both conditions, the red blood cell number has not changed just if they have swollen, or shrunk and that will affect the hematocrit. 04:59 When you think about what is a hypo and a hyper osmotic solution, think of it as the blood. 05:06 If the blood becomes hypo-osmotic, it has a tendency to change the hematocrit by swelling the red blood cells. 05:16 If you're in a hyperosmotic condition of the blood, your red blood cells will tend to shrink, and therefore, your hematocrit will decrease. 05:27 So by knowing the plasma proteins and the hematocrit, we can get a really good idea of where the water might be moving. 05:36 And this will help us to describe the various challenges to body fluid balance. 05:44 Now that we have our ions being measured, we were able to measure our osmolality and measure our oncotic pressures, how do you determine how much is in each fluid compartment? Some of these fluid compartments we can directly measure and other ones we have to calculate. 06:00 So let's go through the first one, which is probably the most important which is plasma volume. 06:05 For plasma volume, we can directly measure this by radioactively tagging albumin, or we can tag it with a dye such as Evans blue dye. 06:15 Then we look at its concentration to determine the plasma volume. 06:20 We can also measure extracellular fluid volume by looking at another radioactive dye with sodium, or we can give a substance like inulin or mannitol. 06:29 And we look at its concentration. 06:32 We can't directly measure interstitial fluid volumes, but we can use is back calculate it from the extracellular fluid compartment minus the plasma volume. 06:43 Finally, we can measure total body water. 06:46 And this is done also through nuclear medicine and where you radioactively tag water. 06:51 And this can be been measured over time to see someone's total water clearance. 06:57 Once you have this particular measurement and the extracellular fluid compartment, you can use that to calculate the intracellular fluid by taking the total body water minus the extracellular fluid volume. 07:10 And this gives us your intracellular fluid. 07:13 Because again, you cannot directly measure intracellular fluid.
About the Lecture
The lecture Measuring Ions and Fluid Compartments by Thad Wilson, PhD is from the course Urinary Tract Physiology.
Included Quiz Questions
Which of the following laboratory measurements is the most important to calculate plasma osmolality?
- Plasma sodium
- Serum glucose
- Blood urea nitrogen
- Albumin levels
Which of the following is part of the basic metabolic profile?
- Creatinine
- ALP
- ALT
- Bilirubin
- Total protein
Which of the following heavily influences oncotic pressure?
- Albumin
- Alpha globulin
- Beta globulin
- Glucose
- Sodium ions
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |