Playlist
Show Playlist
Hide Playlist
Lipids, Fats and Oils
-
Slides 05 MacromoleculesII CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
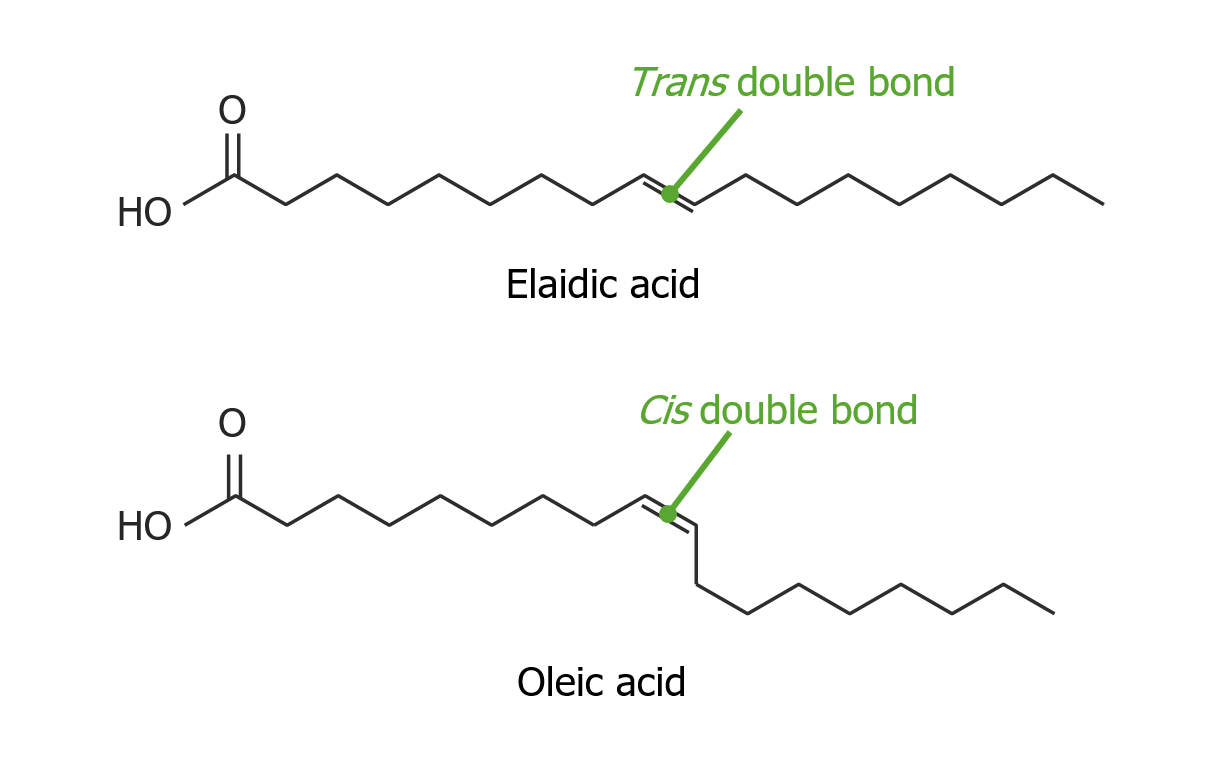
00:01 So now, we’re done with our nucleic acids, we can start looking at lipids. 00:06 Lipids are non-polymer macromolecules. 00:11 Again, we have three different or four different types of lipids. 00:17 We have our fats, we have our oils, that’s sort of one type. 00:21 And then, you have things like terpenes and steroids. 00:24 Terpenes are pigments that we see in plants and steroids are the precursors to many of our hormones and some of the vitamins. 00:31 All of these molecules are non-polymer. 00:35 That is they don’t have repeating subunits of the same sort of thing like glucose molecules are monomers of sugar, or like amino acids are monomers of polypeptides. 00:50 So there are no monomers really to consider even though they do have different subunits, they’re not repeating subunits of the same types of things. 01:00 So first, we’ll take a look at our fats and oils. 01:04 These are the things that we call triglycerides and they are made up of three fatty acid molecules as well as a glycerol molecule to hold them together. 01:17 Some of them will have straight-chain fatty acids, and some of them will have kinky-chain fatty acids, so they’re wonky. 01:25 And this has to do if you’ll recall at the beginning of our lectures on macromolecules, we talked about there being double bonding, we’ll come back to that here shortly. 01:34 So saturated fats are solid at room temperature versus unsaturated fats or our oils, they’re generally liquid at room temperature. 01:46 Butter is a great example of a saturated fat. 01:49 So saturated fats are called saturated because the hydrocarbon chain is fully saturated with hydrogens. 01:58 Each carbon is holding as many hydrogens as it’s possible. 02:02 So it’s covalently bonded to as many hydrogens. 02:06 The other option is it could form some double bonds but this single bonding to as many hydrogens as possible results in having a straight hydrocarbon chain or straight fatty acid tails. 02:18 These straight fatty acid tails lay up on each other really nicely which is why we have solid room temperature. 02:26 If we increase the temperature, we increase molecular motion, and the fat will become more liquid because there’s just more molecular motion. 02:35 On the other hand, we could have oils. 02:38 Oils in general are plant based and they will be liquid at room temperature and they generally have unsaturated or hydrocarbon chains. 02:50 Vegetable oils have generally unsaturated hydrocarbon chains. 02:54 They’re unsaturated because some carbons form a carbon-carbon double bond, therefore, not needing any hydrogens off of the side of the chain. 03:04 Because we’re missing some hydrogens, that allows some flexibility in the fatty acid tails at which point, we see kinky chains in the fatty acid tails. 03:15 Because there are kinky chains at room temperature, these triglycerides do not stack up and so they don’t need a lot of molecular motion to become liquid at room temperature. 03:29 So now we should talk a little bit about you’ve probably heard cyst versus trans fatty acids and what’s the big deal with that really? Are they polyunsaturated, are they monounsaturated? What does this mean? So monounsaturated to start with means that we are missing two hydrogen atoms, resulting in one carbon-carbon double bond. 03:53 So it’s not fully saturated. 03:54 But polyunsaturated means we could be missing several or many hydrogens in the hydrocarbon chain and those are replaced with the carbon-carbon double bonds that allow kinkiness. 04:06 Now, you would think that if we were to add hydrogens to this chain, we could saturate it which is precisely what has happened in the case of margarine. 04:16 So in the 70’s or so, people said, well, you’ve got to have margarine and not butter, margarine’s much better for you, and it was suggested that these saturated fats really had a big impact on HDL cholesterol and LDL cholesterol. 04:31 And eating saturated fats was said to increase LDL cholesterol which is the bad kind of cholesterol and decrease HDL cholesterol which is the good kind of cholesterol. 04:41 We kind of want that to be higher. 04:43 It turns out that there’s been a lot of research done since that sort of refutes the whole hypothesis but in response to that, people started hydrogenating oils in order to make them solid at room temperature and this is where we came up with margarine. 05:00 Well, what’s the problem with margarine? Well, if you think about it, it’s been chemically hydrogenated. 05:06 So we say partially hydrogenated soy bean oil or partially hydrogenated vegetable oil means we’re synthetically adding hydrogens and they don’t have to include the ingredients that they use in order to get those hydrogens to attach to the carbon molecules. 05:22 So I’d say that probably margarine is not the best way to go. 05:27 And it turns out that probably the opposite is the effect. 05:32 That these cis and trans isomers that we see, recall an isomer, is a different arrangement of molecules around the structure of the carbon backbone and a cis transformation is our normal transformation for the bonds to happen. 05:51 In the trans transformation or in the transformation, what we’ll see is that the hydrogens are actually on opposite sides of the hydrocarbon chain, so a different isomeric formation which is somehow not good for us because we don’t have an enzyme to break down that trans-fat. 06:12 And so those trans-fats actually accumulate and they cause an increase in our LDL cholesterol and a decrease in HDL cholesterol which is kind of ironic because that’s exactly what they were saying that saturated fats would cause. 06:29 So margarines not only are they synthetically created and we don’t know what it is that they use to get the hydrogens on there because it doesn’t need to be included in the ingredients, but we’re also having the same effect as was supposed by this 1970’s hypothesis.
About the Lecture
The lecture Lipids, Fats and Oils by Georgina Cornwall, PhD is from the course The Macromolecules of Life.
Included Quiz Questions
Which of the following is NOT correct about lipids?
- Lipids are saturated fats that have long hydrocarbon tails and multiple carbon-carbon double bonds.
- Lipids are nonpolymer macromolecules that are predominantly composed of hydrocarbon chains.
- Fats, oils, waxes, terpenes, steroids, and phospholipids are examples of lipids.
- A fatty acid in the cis configuration has two –R groups on the same side of the carbon-carbon double bond.
- Lipids are structural components that also help in energy storage and cell signaling.
Why are vegetable oils liquid at room temperature?
- The kinkiness in unsaturated hydrocarbon chains does not allow them to stack on top of each other, keeping vegetable oils in a liquid state at room temperature.
- Vegetable oils cannot solidify due to high molecular vibrations in the fully saturated hydrocarbon chains.
- Vegetable oils are liquid at room temperature due to the absence of a metal catalyst.
- Vegetable oils cannot solidify due to low molecular vibrations in the fully saturated hydrocarbon chains.
- Vegetable oils cannot solidify due to the larger number of hydrogen atoms in the fully saturated hydrocarbon chains.
Why are trans fats harmful to health?
- Trans fats increase low-density lipoprotein (LDL) cholesterol levels and lower high-density lipoprotein (HDL) cholesterol levels, increasing the risk of coronary artery disease and metabolism-related disorders.
- Trans fats lower LDL cholesterol levels and increase HDL cholesterol levels, increasing the possibility of brain hemorrhages.
- Trans fats lower LDL and HDL cholesterol levels in the blood, increasing the possibility of liver degeneration.
- Trans fats raise LDL and HDL cholesterol levels in the blood, increasing the risk of liver degeneration.
- Trans fats raise LDL and HDL cholesterol levels in the blood, increasing the risk of intestinal bleeding.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
I loved the correlation with margarine and butter, it helps to understand and remember the topic.