Playlist
Show Playlist
Hide Playlist
Introduction to the Citric Acid Cycle
-
Slides CitricAcidCycle.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
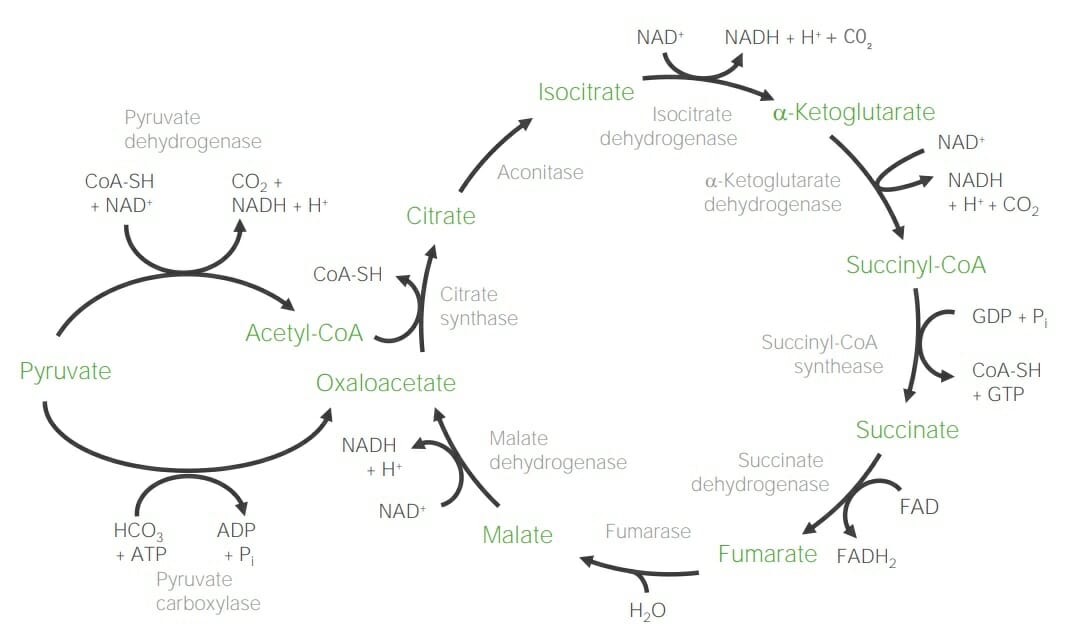
00:01 In this lecture I’ll be talking about pyruvate metabolism, the citric acid cycle, the glyoxylate cycle and then I’ll finish up with ketone body metabolism. 00:11 The process of respiration is essential for cells to be able to convert nutrients into useful energy for the cells to use. 00:19 This process it involves the breakdown of larger molecules into smaller ones. 00:23 Carbohydrates, of course, being broken down into sugars, proteins being broken down into amino acids and fats being broken down into fatty acids and glycerol. 00:33 Now, the central molecule and all these processes is acetyl-CoA as you can see here. 00:39 In this process, Acetyl-CoA feeds the citric acid cycle and as we’ll see later it also feeds into the glyoxylate cycle and the metabolism of ketone bodies. 00:50 Now, the citric acid cycle which I’ll talk about in just a minute is very important for metabolic energy. 00:59 It is in fact the most important oxidative cycle that’s present within cells. 01:04 The citric acid cycle has as its feeding molecules, I noted earlier, the Acetyl-CoA. 01:11 Acetyl-CoA enters the cycle as shown here. 01:14 Now I’ll talk about the reactions in just a couple of minutes, but you can see in this cycle how the citric acid cycle actually interacts with things like amino acids. 01:24 The amino acids shown in blue are those that feed molecules such as acetyl-CoA into the citric acid cycle and the molecules shown in green are those that can be produced for all these acetyl-Coa and other intermediates of the citric acid cycle. 01:41 Now, pyruvate is essential in the process of making acetyl-CoA. 01:47 Acetyl-CoA can be made from it as can be seen on the screen from the process of glycolysis, the breakdown of sugar. 01:54 Now, pyruvate is central to a lot of things happening inside of cells. 01:58 The cycle that you see or this pathway that you see on the screen occurs only when oxygen is present. 02:05 When oxygen is absent, pyruvate does not and in fact cannot go to acetyl-CoA. 02:11 Instead, when oxygen is absent a process called fermentation occurs. 02:17 Now, in animals the process of fermentation produces the molecule known as lactate, as you can see here. 02:24 When that occurs then NAD is produced in order to keep the cells alive. 02:29 Bacteria and yeast on the other hand produce other molecules that allow them to make NAD. 02:36 First, they make the molecule called acetaldehyde as you can see here. 02:40 And then, acetaldehyde is converted into alcohol or ethanol as we know in the process of yeast and bacterial fermentation. 02:49 We’re interested in the process that occurs when oxygen is present and pyruvate is converted into acetyl-CoA. 02:56 This reaction is catalyzed by the enzyme pyruvate dehydrogenase. 03:00 It is a mitochondrial enzyme that has a very large multimeric complex. 03:04 This multimeric complex contains three subunits labeled as E1, E2 and E3. 03:11 Now I’ll talk about those subunits in just a moment but as you can see in the reaction catalyzed by this enzyme, pyruvate on the left is converted down to acetyl-CoA at the bottom of the screen. 03:22 The process occurs slightly differently in bacteria than it does in animals but the overall mechanism the enzyme uses is the same in both organisms. 03:32 Now, the steps in pyruvate oxidation are labeled here and you can see in the labeling that each subunit or the process catalyzed by each subunit is shown individually. 03:43 In this case, the reaction shown here is catalyzed by the subunit known as E1, also known as the pyruvate decarboxylase. 03:51 In this reaction, pyruvate is decarboxylated losing a carbon dioxide and the two molecule intermediate is transferred to a coenzyme known as thiamine pyrophosphate. 04:05 This converts and makes some intermediate called thiamine pyrophosphate acetaldehyde. 04:10 In the next step of the reaction, the thiamine pyrophosphate acetaldehyde transfers the acetaldehyde to a molecule called lipoamide. 04:19 This involves an oxidation as we will see and is catalyzed on the subunit labeled as E2. 04:25 In the last steps of the process, the acetyl group that’s on the acetyl lipoamide is transferred to coenzyme A. 04:33 In the process, electrons are transferred to FAD and ultimately to NAD to form NADH. 04:40 This reaction is catalyzed on the subunit known as E3. 04:44 Now I noted earlier that bacteria use a process that’s slightly different than the animal cells do and that is what actually enables the production of alcohol or ethanol that is involved in bacterial or yeast fermentation, and that’s illustrated here. 05:00 In this step of the process, the acetaldehyde group from the thiamine pyrophosphate acetaldehyde is released as free acetaldehyde and that does not occur in animals and that’s why we can’t ferment or make our own alcohol in our bodies. 05:15 That acetaldehyde then becomes the substrate to make ethanol. 05:19 That does not have to happen and in fact it only happens in bacteria and yeast when oxygen is absent. 05:26 We don’t have that ability in animals to do that. 05:29 Now, the overall mechanism is shown in this rather complicated slide that you can see here. 05:36 I’m going to step you through the process to try and illustrate the important reactions that are occurring within the pyruvate dehydrogenase complex. 05:45 They’re labeled in four steps as A, B, C and D and the subunit where the reactions are occurring is labeled as E1, E2, E3 and E4. 05:58 As noted earlier in the first step of the process, pyruvate is decarboxylated losing the carbon dioxide and the two molecule intermediate known as acetaldehyde is transferred to thiamine pyrophosphate. 06:12 Now the structure of thiamine pyrophosphate is shown above the arrow labeled A. 06:18 You can see the intermediate that’s shown in the reaction after A. 06:22 In the next step of the process, the acetaldehyde that is on the thiamine pyrophosphate is transferred into a molecule called lipoamide within the subunit called E2. 06:37 Now this step of the process is the step in which the oxidation actually occurs. 06:43 This oxidation involves a reduction because every oxidation of one molecule involves the reduction of another. 06:51 In this case, the acetaldehyde is becoming oxidized to an acetyl group, as you can see, after reaction B up there. 06:59 And what has happened is a disulfide bond shown in the lipoamide underneath the B that is the sulfur-sulfur bond; that disulfide bond is broken by the reduction of the disulfide bond with electrons coming from acetaldehyde that results in the production of an acetyl group and the structure that you see on the right. 07:22 This process occurs, of course, occurs on subunit E2. 07:27 Now the last steps in the process occur within the subunit labeled E3. 07:32 In this process, first of all, the acetyl group is transferred from the lipoamide molecule to another coenzyme known as coenzyme A and we can see that occurring in step C. 07:45 In this process, acetyl-CoA, the final product of the overall process is produced and it’s released. 07:54 One problem though is that the lipoamide which started out as a disulfide bond is now in the reduced or sulfhydryl form. 08:04 The electrons from the sulfhydryls must be transferred to electron carriers to reform the disulfide bond and that occurs in steps D and in step E as you can see here. 08:17 First, the electrons are transferred to FAD to form FADH2 and then FADH2 transfers electrons to NAD to form NADH, completing the overall process and regenerating the oxidized lipoamide. 08:32 The lipoamide in the oxidized and reduced forms that I described in the previous slide are shown here in more detail. 08:39 The top form shows the lipoamide in the oxidized form. 08:43 Now note that we call the molecule lipoamide when lipoic acid, the smaller component, is covalently attached to a protein. 08:52 That occurred in the last slide when we have lipoamide made from lipoic acid attached to the subunit E2. 09:00 The oxidized form of lipoic acid is shown on top and we can see that it’s oxidized by virtue of the fact that the two sulfurs on the left side of the slide have a covalent bond between them. 09:12 Reducing that bond of the disulfide with electrons from acetaldehyde as occurs in E2 produces sulfhydryl bonds that you can see on the lower left and now the two sulfurs are not covalently joined to each other. 09:29 The acetyl-CoA is a very complicated molecule. 09:32 Most of the portion of the molecule, the coenzyme A part, is shown on the right side of the slide in black and you can see it’s a fairly large molecule and the part of interest to us is shown only in the green on the left. 09:46 So that’s why when I refer to acetyl-CoA I will commonly say that it’s a two carbon molecule, although there are many more carbons in the molecule, the only parts being transferred are the two carbons of the acetyl group.
About the Lecture
The lecture Introduction to the Citric Acid Cycle by Kevin Ahern, PhD is from the course Carbohydrate Metabolism. It contains the following chapters:
- Introduction to the Citric Acid Cicle
- Ketone Body Metabolism
Included Quiz Questions
Which of the following molecules is central to most of the biological reactions in the cell during metabolism?
- Acetyl CoA
- Ethanol
- Glucose
- Lipoamide
- Acetaldehyde
Which of the following amino acids is not synthesized from α-ketoglutarate of the citric acid cycle?
- Tyrosine
- Glutamine
- Glutamate
- Proline
- Arginine
Which of the following statements is not true regarding the citric acid cycle?
- The citric acid cycle enables the yeast or a bacterial cell to get metabolic energy from acetyl Co-A in the absence of oxygen.
- The catabolic breakdown of amino acids produces the intermediates of the TCA cycle.
- During anabolism of amino acids, the intermediates of the TCA cycle are used to synthesize the amino acids like glutamine, glutamate, proline, and arginine.
- The oxaloacetate, a TCA cycle intermediate, acts as a precursor for the synthesis of aspartate, asparagine, methionine, and threonine.
- In the absence of oxygen, the acetyl Co-A can’t enter the TCA cycle, so the yeast produces energy by converting pyruvate to ethanol.
Which of the following statement is not true regarding the pyruvate dehydrogenase enzyme?
- The pyruvate dehydrogenase enzyme converts the acetaldehyde to ethanol in the yeast cell in the absence of oxygen.
- The pyruvate dehydrogenase enzyme is a mitochondrial enzyme.
- The pyruvate dehydrogenase enzyme is a very large multimeric complex composed of three subunits named E1, E2, and E3
- The E2 subunit of pyruvate dehydrogenase enzyme converts the TPP-acetaldehyde to acetyl-lipoamide, whereas the E3 subunit coverts the acetyl-lipoamide to acetyl-CoA.
- The enzyme pyruvate dehydrogenase converts the pyruvate to acetyl Co-A via decarboxylation in the presence of oxygen.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |