Playlist
Show Playlist
Hide Playlist
Glycolysis: GAP –> 1,3 BPG – Glycolysis and Pyruvate Metabolism
-
04 Advanced CarbohydrateMetabolism.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
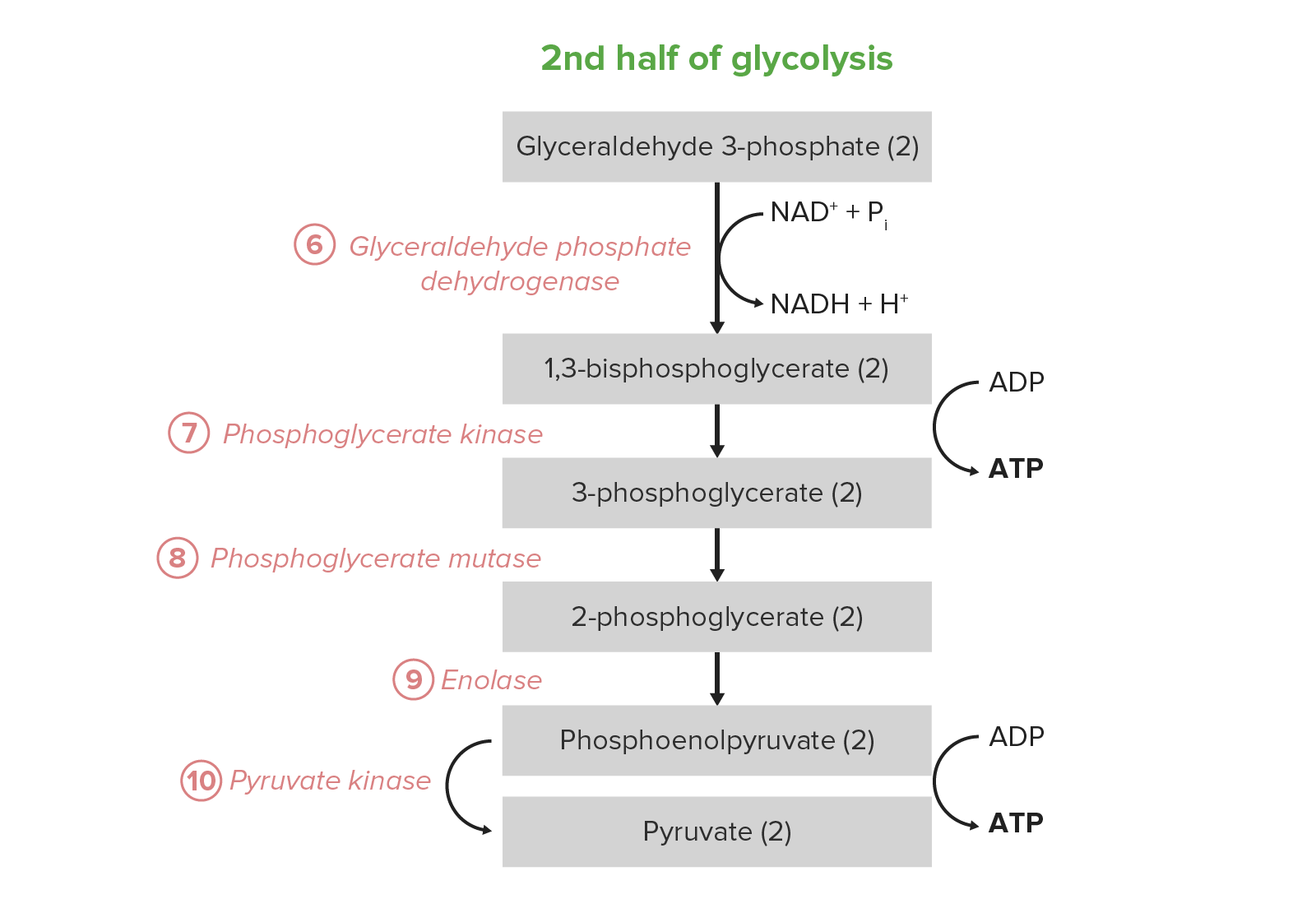
00:01 In the next reaction and I wanna remind you that from this point forward everything will be doubled. So we actually have two glyceraldehyde-3-phosphate and we are gonna produced 2 molecules of 1,3-bisphosphoglycerate. 00:14 Now this reaction is important because it is the only oxidation that occurs in glycolysis. 00:20 We think about glycolysis as being a pathway this very oxidated, but in-fact it's not. There is only one oxidation that occurs. 00:28 We think about glycolysis being oxidated because of the product that later get oxidized in other pathways. 00:35 This reaction is an interesting one. Okay? We see for example on the left we have an aldehyde and on the right we have an ester. 00:42 That's the indication that we have had in oxidation. 00:45 The delta G zero prime for this reaction is a little positive. It's 6.3 kJ/mol but again the cell doesn't have a big problem with that. 00:54 The difference here is that if we look at what's happening in this reaction, a phosphate is being added directly onto the acid to make the ester, on the right side. 01:05 When we did a similar reaction for glucose going to glucose-6-phosphate we had to couple that to ATP. 01:12 And the same thing happened when we went fructose-6-phosphate to fructose-1,6 bisphosphate. 01:18 We had to use the energy of ATP. So why don't we have to use the energy of ATP here? The answer is because we have an oxidation. Remember that oxidative reactions generate energy and the energy of this oxidation is helping to favor this reaction going forward. 01:34 The oxidation of course generates electrons so we see NAD+ becoming NADH.
About the Lecture
The lecture Glycolysis: GAP –> 1,3 BPG – Glycolysis and Pyruvate Metabolism by Kevin Ahern, PhD is from the course Carbohydrate Metabolism.
Included Quiz Questions
Which of the following is true of glyceraldehyde-3-phosphate?
- It is the only molecule in glycolysis that gets oxidized.
- It is made in glycolysis from 1,3-bisphosphoglycerate (1,3 BPG).
- It requires NADH to be oxidized.
- It requires ATP to be oxidized.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |