Playlist
Show Playlist
Hide Playlist
Four Levels of Organization
-
Slides 05 MacromoleculesII CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
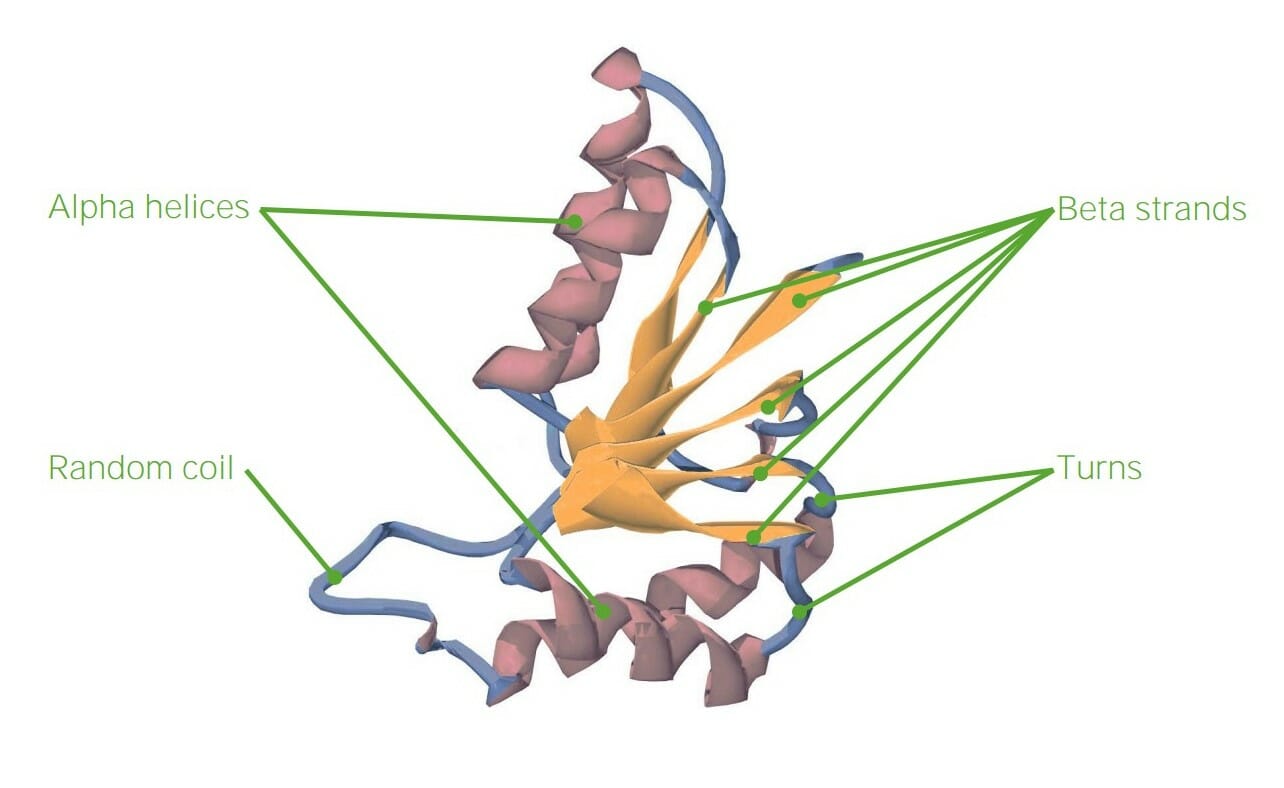
00:00 So beyond the primary structure which is the sequence of amino acids, we have secondary, tertiary, and quaternary levels of structure for our proteins. 00:13 So again, primary structure is simply the amino acid sequence. 00:18 Which amino acid with their distinct side groups is linked to the next amino acid? One might move up a level of organization in structure of proteins, we get to secondary structure in which we see two distinct patterns of bonding. 00:34 This involves hydrogen bonding between the carboxyl group of one amino acid and the amino group of another. 00:42 In the case of the alpha helix, these are about four amino acids away from each other but that hydrogen bonding between those groups allows the formation of this alpha helix. 00:53 Another formation that we see commonly in secondary structure are beta pleated sheets. 01:00 The amino acids sort of associate and form this step-like structure and again, the carboxyl groups of one end of an amino acid and the other end in an antiparallel piece will connect in order to hold the beta pleated sheets together. 01:18 We see these structures repeatedly throughout any protein molecule. 01:24 When we move up a level from there, we see tertiary structures. 01:28 So we have a long polypeptide chain, single amino acids. 01:32 In addition to that, we might have alpha helices or beta pleated sheets and then, tertiary structure is where we see association between different side groups, so the R-groups of the amino acids. 01:48 The secondary structure was hydrogen bonding between the carboxyl and amino groups. 01:53 Here, we’re seeing the R-groups interacting. 01:56 So tertiary structure can be formed by a number of different interactions. 02:02 First of all, we could have hydrophobic exclusion. 02:06 Meaning, hydrophobic meaning that it does not like water. 02:11 So in general, when we have proteins in solution for example, hemoglobin, that wants to hold on to oxygen, then it will create a hydrophobic region in which we can have oxygen bind, right, or a specific domain in which that oxygen can bind. 02:32 Another thing we might see is interaction of R-groups where we actually have an ionic bond forming because one is positively charged and one is negatively charged. 02:43 And again, we could also see disulfide bridges. 02:47 So we have sulfur involved in those. 02:49 Those are some of the most strong bonds that we’ll see in proteins. 02:54 So as you can see, the shape of an amino acid chain folding into its protein is determined by the types of R-groups that we see associated with that central carbon. 03:09 Quaternary structure is not exhibited in all proteins. 03:14 We definitely see primary, secondary, and tertiary structure in every protein. 03:20 However, a quaternary structure involves association of multiple different polypeptide chains. 03:26 Hemoglobin is a great example and we’ll come back and visit hemoglobin throughout the rest of the course, but it has four subunits. 03:35 Two alpha subunits, and two beta subunits. 03:38 And those are associated together and drawn together by charges again with the R-groups. 03:46 So when we consider tertiary and quaternary structure, there are several different repetitive folding patterns that we see or motifs. 03:55 It’s very common to see a beta pleated sheets with an alpha helix in the middle and another series of beta pleated sheets which we call a beta alpha beta motif. 04:07 We also might see a helix turn helix motif. 04:12 And beta barrels are fairly common as we see in membrane channels. 04:18 As you can see these different motifs probably end up causing different regions of the protein to have different functions. 04:27 Those different regions of a protein can be called domains. 04:31 So one domain might have a function in binding to a membrane while another domain has a function in binding to a ligand, and the other domain may interact with the G protein, for example, and we’ll explore how those different domains interact once we put together the cells and get some membrane structures, and talk about cell signaling. 04:52 Another thing that helps in folding proteins, sometimes proteins become denatured and we might need to refold them are the chaperone proteins. 05:03 Chaperone proteins themselves are proteins with multiple subunits. 05:07 However, they sort of act like a barrel and the barrel will open up and take in a polypeptide chain that’s been misfolded. 05:17 It may require a little bit of ATP to fuel the process, give it energy, so that this chaperon protein or chaperonin as we call it can then help the protein refold into its shape and release it back into the environment that it lives in perhaps, the cell. 05:35 And so these chaperone proteins, we don’t understand entirely how they work yet, but we do know that they exist in helping proteins associate to form their shape. 05:47 Because if you didn’t have chaperone proteins, how would you particularly get one polypeptide chain to associate this positive group with that negative group. 05:58 So we know there are assistants along the way and this is what we’ve learned about so far. 06:03 So when a protein is misfolded, it could become denatured. 06:07 Denatured means that it’s no longer functional. 06:10 It unfolds, it no longer binds with the particular membrane section that it needs to or it no longer binds with the ligand that it needs to. 06:20 A number of different things can cause this denaturation and that would include things like changes in homeostasis, for example, pH change could cause the proteins to unfold. 06:32 When we think about polar R-groups, those polar R-groups are interacting with each other depending on the acidity or basicity of the environment. 06:42 So when we change pH, sometimes those R-groups are no longer interested in each other at all. 06:48 Temperature is another variable that can affect how a protein folds or denatures. 06:54 Temperature is a great example when we fry an egg. 06:57 The protein is in one form. 07:00 When we add heat, the proteins change form and we can generally not reverse frying an egg. 07:07 So all these changes or denaturations of protein are generally irreversible which is where chaperone proteins can come in helpfully in cells. 07:16 Another impact is ionic concentration. 07:20 If we change the ionic concentration inside a cell, that could also cause the R-groups of a protein to dissociate because they’re no longer interested in each other. 07:31 If the environment becomes particularly positive inside the cell as in lots of positive ions, then we’ll see the R-groups dissociate as we would if it becomes particularly negative.
About the Lecture
The lecture Four Levels of Organization by Georgina Cornwall, PhD is from the course The Macromolecules of Life.
Included Quiz Questions
Which of the following can cause proteins to denature? Select all that apply.
- Changes in ion concentration
- Changes in pH
- Changes in temperature
- A stable environment
- Chaperones
Which of the following is paired INCORRECTLY with respect to the structural organization of proteins?
- Quinary structure – association between the –R and carboxyl groups of different amino acids
- Primary structure – sequence of amino acids joined via peptide bonds
- Tertiary structure – associations between the –R groups of various amino acids in a peptide chain
- Quaternary structure – association of different peptide chains
- Secondary structure – hydrogen bonds between the carboxyl and amino groups of different amino acids
Which of the following best describes a beta-pleated sheet?
- The wavy sheet-like appearance due to the linking of two or more beta sheets via hydrogen bonding
- The wavy sheet-like appearance due to the linking of two or more alpha-helices via disulfide bonds
- The wavy sheet-like appearance due to hydrophobic interactions between two or more beta sheets
- The wavy sheet-like appearance due to hydrophobic interactions between two or more alpha-helices
- The wavy sheet-like appearance due to the linking of two or more beta strands via ionic bonds
Which statement is NOT true regarding the tertiary structure of proteins?
- When the four subunits of hemoglobin come together, they form a tertiary structure.
- The tertiary structure of proteins involves the associations between the –R groups of amino acids.
- –R groups determine the shape of an amino acid chain folding into its protein.
- Disulfide bridges, ionic interactions, and hydrophobic interactions play a significant role in stabilizing the tertiary structure of proteins.
- The tertiary structure of proteins may involve repetitive patterns of motifs or domains.
Which of the following best describes the quaternary structure of a protein?
- A complex of two or more polypeptide chains held together by noncovalent forces
- A complex of two or more amino acids held together by covalent bonds
- A complex of two or more amino acids held together by noncovalent bonds
- A complex of two or more polypeptide chains held together by covalent bonds
- A complex of two or more polysaccharide chains held together by covalent bonds
Customer reviews
3,5 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
0 |
I saw many times the video, take notes but I don't understand at all this video; think is some confusing :(
amazing lecture, finally i understood the tertiary structure for the first time