Playlist
Show Playlist
Hide Playlist
Fatty Acids – Lipids
-
08 Basic Lipids-Fats&Oils.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
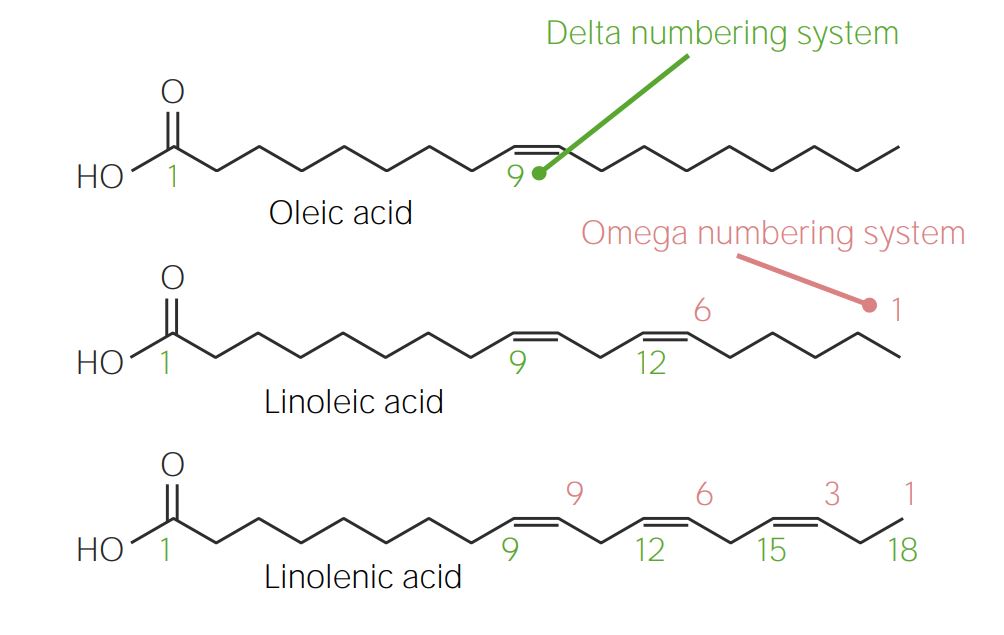
00:00 Eines der interessanten Dinge an der Ungesättigtheit einer Fettsäure sind ihre Eigenschaften. 00:08 Wir können zum Beispiel sehen, wenn wir die einzelnen gesättigten Fettsäuren vergleichen, also Myristinsäure, Palmitinsäure und Stearinsäure, der Schmelzpunkt dieser Fettsäuren steigt, wenn wir von 14 auf 16 auf 18 Kohlenstoffe erhöhen. 00:24 Wenn wir nun die gleichen 18 Kohlenstoffe nehmen und sie mit ungesättigten Fettsäuren vergleichen, wie man hier sehen kann, haben alle diese Fettsäuren 18 Kohlenstoffe, sie unterscheiden sich in der Anzahl der Doppelbindungen, die sie enthalten. Die Stearinsäure ohne Doppelbindungen hat einen Schmelzpunkt von 71°C, während Linolensäure mit drei Doppelbindungen einen Schmelzpunkt von -11°C hat. 00:48 Offensichtlich reduziert die Entsättigung die Schmelzpunkte der Fettsäuren und reduziert folglich den Schmelzpunkt der Fette, die diese Fettsäuren enthalten. 00:58 Ich habe die Struktur der ungesättigten Fettsäuren beschrieben, aber jetzt können Sie drei von diesen Strukturen hier auf dem Bildschirm sehen. Ölsäure, Linolsäure und Linolensäure unterscheiden sich durch die Anzahl der Doppelbindungen, die sie enthalten. Sie können sehen, dass Ölsäure eine Doppelbindung auf Position Nummer 9 hat. Wenn wir nun die Position von Doppelbindungen innerhalb von Fettsäuren beschreiben, gibt es zwei verschiedene Nummerierungssysteme, die verwendet werden. 01:24 Das erste Nummerierungssystem wird als Delta Nummerierungssystem bezeichnet und es ist dasjenige, das oben für die Ölsäure beschriftet ist. Das Delta-Nummerierungssystem nummeriert Kohlenstoff Nummer eins als die Carboxyl-Gruppe am Ende des Moleküls, dann zählen wir neun Kohlenstoffe nach innen und kommen zur Doppelbindung, die sich in der Ölsäure befindet. In Linolsäure sieht man sowohl die Nummerierung eins bis zwölf unten in grün als auch die Nummerierung von rechts nach links. 01:54 Die Nummerierung von rechts nach links wird Omega-Nummerierungssystem genannt, es beginnt bei der Kohlenstoffzahl eins mit der Methylgruppe am Ende der Fett- säure. Linolsäure wird als eine Omega-6-Fettsäure bezeichnet und hat biologisch eine etwas andere Wirkung auf den Körper im Vergleich zu anderen Omegafettsäuren. Linolensäure ist die Fettsäure, die als Omega-3-Fettsäure bezeichnet wird. 02:24 Omega-3-Fettsäuren werden in vielen Fällen damit in Verbindung gebracht, was die Menschen für gut halten, was die Gesundheit fördert, was gut fürs Herz ist und so weiter. Das Omega-System und das Delta-System unterscheiden sich voneinander und es ist wichtig, das zu erkennen. Einer der Gründe, warum wir das Delta-Nummerierungssystem haben, also mit dem wir die Position von Doppelbindungen beschreiben, die von bestimmten Organismen hergestellt werden können, ist, weil der Positionsunterschied zur Carboxylgruppe entscheidend dafür ist, ob die Enzyme in einem Organismus funktionieren können. So hat zum Beispiel Ölsäure die Bindung an Position neun, wie Sie hier sehen können, und diese Bindung kann von tierischen Zellen synthetisiert werden. Im Gegensatz dazu können tierische Zellen nicht die Bindungen an der Position zwölf oder an Position fünfzehn synthetisieren. Und deshalb werden Linolsäure und Linolensäure als essentielle Fettsäuren bezeichnet. Mit der Nomenklatur, die hier verwendet wird, um diese Fettsäuren zu beschreiben, können Sie hier auf dem Bildschirm sehen, dass Ölsäure als einfach ungesättigt beschrieben wird, weil es nur eine einzige Doppelbindung hat. Linolensäure und Linolsäure werden beide als mehrfach ungesättigt bezeichnet, weil sie mehr als eine Doppelbindung haben. 03:41 Die Namen, die wir zur Beschreibung von Fettsäuren verwenden, werden auch für die Fette verwendet, die sie enthalten. 03:47
About the Lecture
The lecture Fatty Acids – Lipids by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following is true regarding the melting temperature of fatty acids?
- it is lower for essential fatty acids than a fatty acid with one double bond.
- It increases as saturation decreases.
- It increases as unsaturation increases.
- A saturated fatty acid with 18 carbons has a lower melting point than an unsaturated fatty acid with 18 carbons.
Why does linolenic acid have a lower melting temperature than stearic acid even though both have 18 carbon chain lengths?
- The increase in unsaturation in the fatty acids leads to a reduction in melting temperatures.
- The increase in saturation in the fatty acids leads to a reduction in melting temperatures.
- The increase in single bonds in the fatty acids leads to a reduction in melting temperatures.
- The increase in double bonds in the fatty acids leads to an increase in melting temperatures.
- The increase in triple bonds in the fatty acids leads to an increase in melting temperatures.
Which of the following statement is not true?
- Animal cells can form double bonds at positions 12 and 15 in the fatty acids.
- The increase in the chain lengths leads to an increase in the melting temperatures for saturated fatty acids.
- The delta numbering system and omega numbering system are used to determine the positions of double bonds in the fatty acids.
- The omega-3 fatty acids are considered good fatty acids for the health of the heart.
- The omega numbering system starts numbering the carbons from the methyl group.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |