Playlist
Show Playlist
Hide Playlist
Enzymes: Activation Energy and Mechanisms — Enzyme Catalysis
-
01 Advanced Enzymes&Kinetics1.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
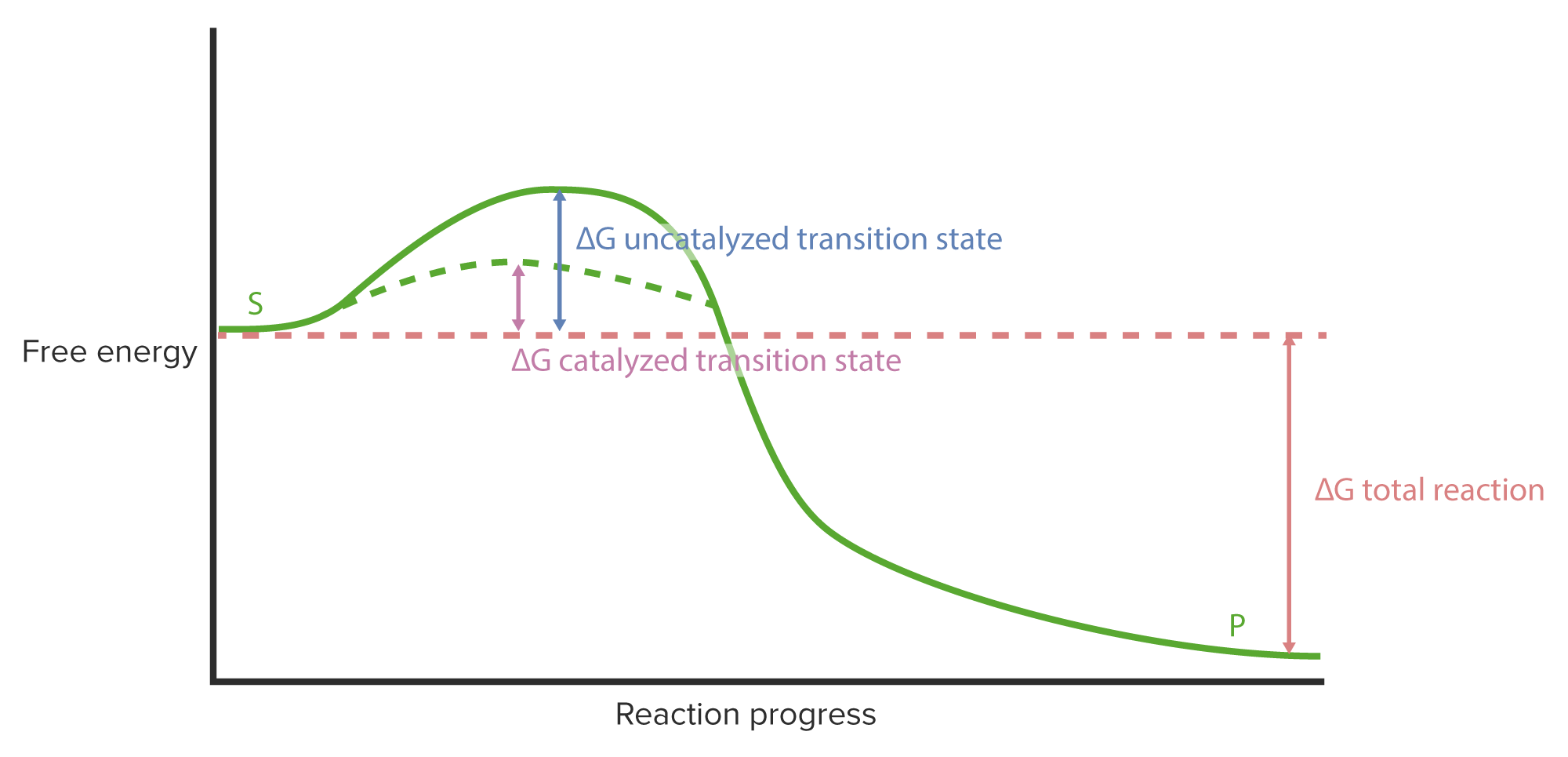
00:00 Wir haben gelernt, wie Enzyme über ihre Flexibilität ihre einzigartige Funktion ausüben. 00:06 Aber Enzyme haben Beschränkungen, unter denen sie arbeiten müssen. 00:09 Ich habe in meinen Präsentationen bereits mehrfach erwähnt, dass Enzyme und Zellen den Regeln des Universums unterliegen. Das heißt, sie können nicht einfach die Energien von Reaktionen verändern. 00:21 Das gilt für Zellen und auch für Enzyme. 00:23 Enzyme, wie wir sehen werden, sind raffiniert. Ich habe erwähnt, wie Enzyme schummeln und Enzyme schummeln auch in Bezug auf Energie. 00:33 Betrachten wir also eine Reaktion von A nach B. 00:36 Wenn A in B umgewandelt wir, können wir die Energie auftragen, wie Sie auf dem Bildschirm sehen. 00:41 Auf der linken Seite des Bildschirms sehen wir einen Punkt auf dem Diagramm, der freie Energie repräsentiert. 00:44 Das ist die Energie von Molekül A. 00:50 Auf dem Weg von A nach B sehen wir, dass es sich die Energie ändert, dass die Energie tatsächlich erhöht. Wir nennen diesen Anstieg "Aktivierungsenergie". Diese muss aufgebracht werden, um eine Reaktion hervorzurufen. 01:02 Die Reaktion schreitet voran und während die Reaktion abläuft, können wir sehen, dass die freie Energie sinkt und wir ein Produkt B erhalten, das letzendlich eine niedrigere freie Energie als A besitzt. 01:14 Das bedeutet, dass Energie auf dem Weg von A nach B freigesetzt wurde und das macht diesen Reaktionsprozess günstig. 01:24 Jetzt ist es wichtig, zu verstehen, dass diese Änderung der freien Energie, die hier gezeigt wird, nicht durch ein Enzym verändert werden kann. 01:34 Das heißt, es gibt keine Veränderung zwischen dem Anfangs- und Endpunkt des Enzyms. 01:38 Das Enzym kann dafür aber andere Dinge tun. 01:42 Es ist wichtig zu beachten, dass dieser Gipfel einen wirklich kritischen Punkt darstellt. 01:46 An diesem Gipfel kann die Reaktion umkehren und von dort, wo sie herkommt, wieder zurückgehen. 01:51 Das heißt, A kann starten und dann wieder zurückgehen. 01:54 Oder B könnte, sofern es genug Energie hätte, diese Kurve erklimmen und dann zu A zurückkehren. 01:58 Andernfalls bewegt sich A nach B und die Reaktion findet statt. 02:08 Nun, Enzyme schummeln, okay? Enzyme können die Aktivierungsenergie verändern. 02:16 Es gibt keine Regeln für die Aktivierungsenergie, okay? Es gibt Regeln für die Anfangs- und Endenergie. 02:19 Die Veränderung der Aktivierungsenergie bewirkt, dass ein Enzym mehr Molekülen diesen Übergang erleichtern kann. 02:32 Das ist die Magie der Enzyme. Wie erreichen sie das? Nun, sie erreichen das auf verschiedene Art und Weise. 02:36 Dies geschieht unter anderem durch die Tatsache, dass sie Bindungsstellen haben, die sehr genau ausgerichtet sind. Die Moleküle liegen so nahe beieinander - zufällig würden sie das nicht tun, richtig? Und das bedeutet, dass sie weniger Energie benötigen, um den nächsten Schritt im Prozess zu gehen. 02:54 Auf diese Weise können die Enzyme die Übergangsenergie senken. 03:01 Nun, wenn die Übergangsenergie niedriger ist, wird es für A viel einfacher, nach B zu gehen, wie wir gesehen haben. 03:07 Sie sehen wieder, dass die Enzyme keine Änderung der gesamten freien Energie erfahren haben. 03:13 Die Energie von A ist immer noch A. Die Energie von B ist immer noch B, okay? Nur dieser Übergangszustand hat den Ausschlag gegeben. 03:20 Ich möchte nun etwas Zeit darauf verwenden, über den Mechanismus einer enzymatischen Reaktion sprechen. 03:27 Es ist wichtig, den Mechanismus zu betrachten, denn, darüber können wir begreifen, wie Enzyme die Elektronenveränderungen ermöglichen, die für das Zustandekommen einer chemischen Reaktion notwendig sind. 03:39 Das Beispiel, das ich verwenden werde, ist das Beispiel einer Serinprotease. 03:41 Serinproteasen sind eine Klasse von Enzymen, die Proteine zerschneiden. Sie spalten Peptidbindungen. 03:50 Das ist es, was sie tun. Sie spalten nicht jede Peptidbindung, die sie sehen. 03:54 Aber sie spalten ganz bestimmte Peptidbindungen an spezifischen Stellen innerhalb der Proteine, an die sie binden. 03:59 Nun gut. Das bedeutet also, dass sie eine Bindungsspezifität haben. Sie zerschneiden nicht alles, was sie sehen. 04:05 Serinproteasen sind flexibel. In der Abbildung am Anfang haben wir die Flexibilität von Enzymen gesehen. Wir werden dem hier wieder begegnen, wenn wir über den Mechanismus der Serinprotease sprechen. 04:19 Die elektronische Umgebung ist entscheidend für eine Reaktion. 04:23 Bei einer chemischen Reaktion werden Elektronen manipuliert, Elektronen werden verschoben. 04:26 Und um das tun zu können, braucht man eine geeignete Umgebung für diese Elektronen, sodass sie sich leicht bewegen können. 04:33 Und wir werden sehen, dass genau dies im aktiven Zentrum der Serinprotease geschieht. 11:14 Enzyme verwenden auch Coenzyme. 04:40 In diesem Beispiel werde ich kein Coenzym zeigen. Aber ich sage Ihnen: Coenzyme helfen einem Enzym, zu funktionieren. 04:51 Okay. Nun, Serinproteasen spalten wie gesagt Peptidbindungen. Das ist die katalytische Aufgabe, die sie erfüllen.
About the Lecture
The lecture Enzymes: Activation Energy and Mechanisms — Enzyme Catalysis by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
In enzyme catalysis, which of the following statements is NOT true?
- Catalysis only occurs in reactions with more than one substrate.
- The enzyme in the ES complex has a different structure than the enzyme alone.
- The binding of substrate induces a change in enzyme structure.
- As a product is released, the enzyme reverts to its original structure.
How do enzymes catalyze chemical reactions?
- By lowering the activation energy of the reaction.
- By lowering the free energy of the products.
- By lowering the free energy of the reactants.
- By increasing the activation energy of the reaction.
- By increasing the free energy of the reactants.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Recomendo pra todos que querem saber melhor sob Medina das universidades