Playlist
Show Playlist
Hide Playlist
Electrochemical Gradient
-
Slides 17 KrebsETCChemiosmosis CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
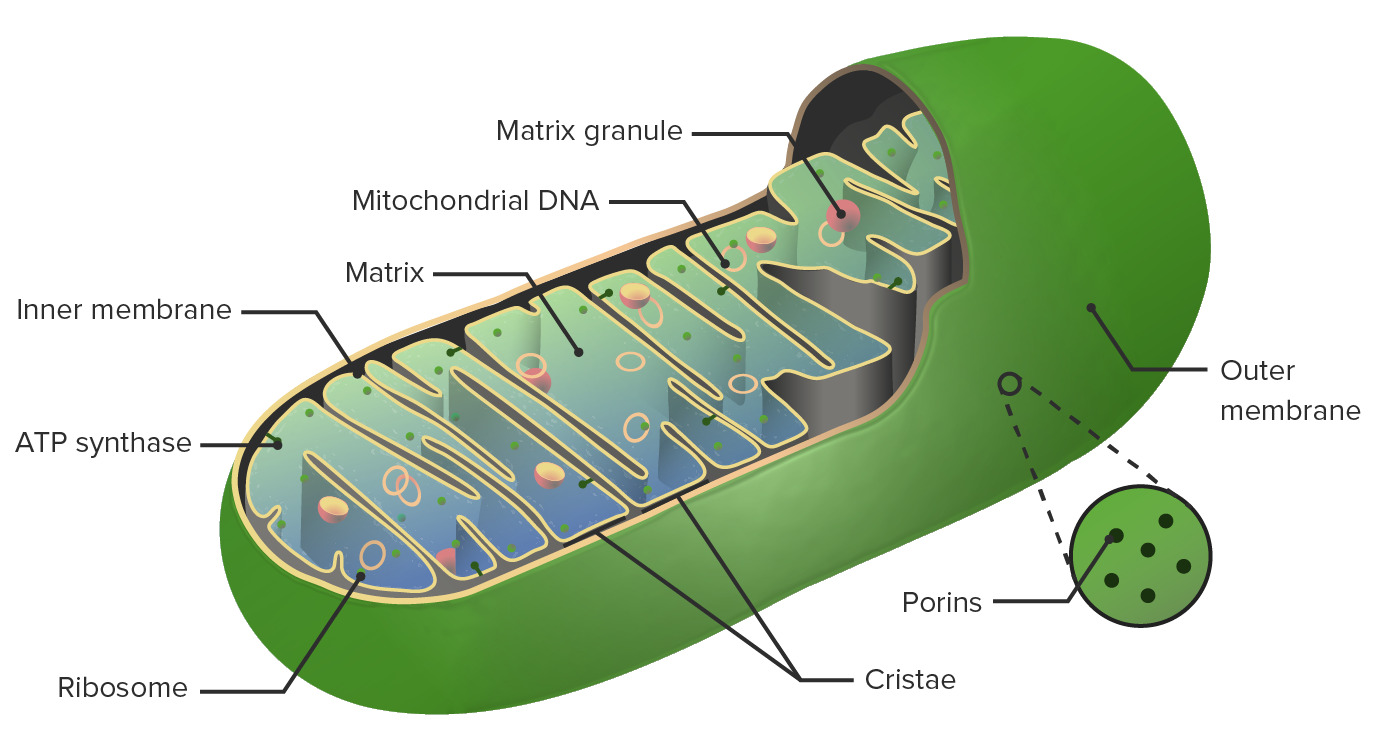
00:00 So breaking that down a little bit more, let's look at how this electrochemical gradient is created. 00:06 We have a number of different electron acceptors embedded together in the inner mitochondrial membrane. 00:16 So you'll recall the mitochondria has two membranes. An external membrane and then a convoluted internal membrane. And we are going to be pumping hydrogens into that tight space. 00:27 So it's much easier to create a gradient in that way. So, what will happen first is the electrons will be picked up by the first electron acceptor. Now, this is where I introduced the idea between NAD taxis and FAD budget taxis. The NAD taxis will receive electrons at a higher level in the staircase. 00:53 I imagine these electron acceptors as a staircase and the electrons are simply bumping down the staircase each time releasing a little bit of energy. The energy that they release is used to pump hydrogen ions across into the intermembrane space. So, FAD drop off their electrons at a slightly lower level. 01:18 And then that acceptor molecule will pass them on to the next molecule, and the next molecule, and so on and so forth bumping them down the chain until oxygen is the final electron acceptor. 01:32 Now we say its a 1/2 O2 because in the end we're going to generate water, and water has H2 and 1O, but also, it's one of the few places that we see oxygen not in the form of O2. Oxygen is separated and becomes very electronegative. Cause you'll recall it really wants to bond with another oxygen. 01:59 So it becomes very electronegative and in this sense, that electronegativity, that very strong draw for electrons is what pulls the electrons down this energy staircase. The 1/2 O2 is on the inside in the mitochondrial matrix. It's going to grab those electrons and hold on to them for a moment. 02:26 Let's look at where the H's come from. The H's are being pumped across into the intermembrane space, and now we have a strong desire for them to come back in. Well, let me continue a moment with my analogy of the prom or the dance we've gone to. Things get a little bit complicated. The taxis have dropped off all of the girls and the boys at this point at the top of the electron transport chain. It's a staircase. 02:54 There is a staircase that goes down into the dance. And the door guy, the guy that's running the door, the first electron acceptor says "Hey ladies, welcome. Come on down the stairs", and he lets all the ladies walk down the stairs. So the electrons happily make their way down into the dance and are having a great time chatting with each other. Meanwhile, this bouncer, the door guy is not such a nice guy to the protons, the hydrogens. So he throws them out the back door into the alley or the intermembrane space. 03:28 He throws them out into the alley. Now they're back there and they are angry. And there is lots of them back there. They are so upset and they are going looking for a doorway to come back in and meet up with the girls. 03:43 So, that's the situation. 03:46 So now that we have created this big gradient, we have to get the hydrogens back into the dance next to their electrons to have a great time. But in this process, we are going to make tons of energy. 03:59 So this is where all the pieces come together to form lots and lots and lots of ATP. Huge yields.
About the Lecture
The lecture Electrochemical Gradient by Georgina Cornwall, PhD is from the course Energy, Enzymes and Metabolism.
Included Quiz Questions
As electrons move along the electron transport chain, they lose potential energy. How is the released energy used by cells?
- The energy is used to transport protons against their concentration gradient.
- The energy is used to pump electrons along the electron transport chain.
- The energy is converted directly into ATP.
- The energy is used to pump NAD+ into the cytoplasm so that it can be used for glycolysis.
- The energy is used to power lactate fermentation.
What is the final electron acceptor in the ETC?
- Oxygen
- Water
- CO2
- Hydrogen
- NAD+ or FAD
Customer reviews
4,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
0 |
As much as your stories are interesting for the ETC gradient It does not fit my learning style. It would be nice to have clear scientific language about what's happening. I can't write a story on my exam paper. Perhaps this is just me.
Really great explanations of the whole process. Was not happy that I had to relearn Krebs and ETC but actually found these videos enjoyable and valuable.
Gooood job LoL and really a good story-maker????Thank you very much! Amazing, very impressive!