Playlist
Show Playlist
Hide Playlist
Competitive Reversible Enzyme Inhibition – Enzyme Inhibitors
-
03 Advanced Enzymes&Kinetics3.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
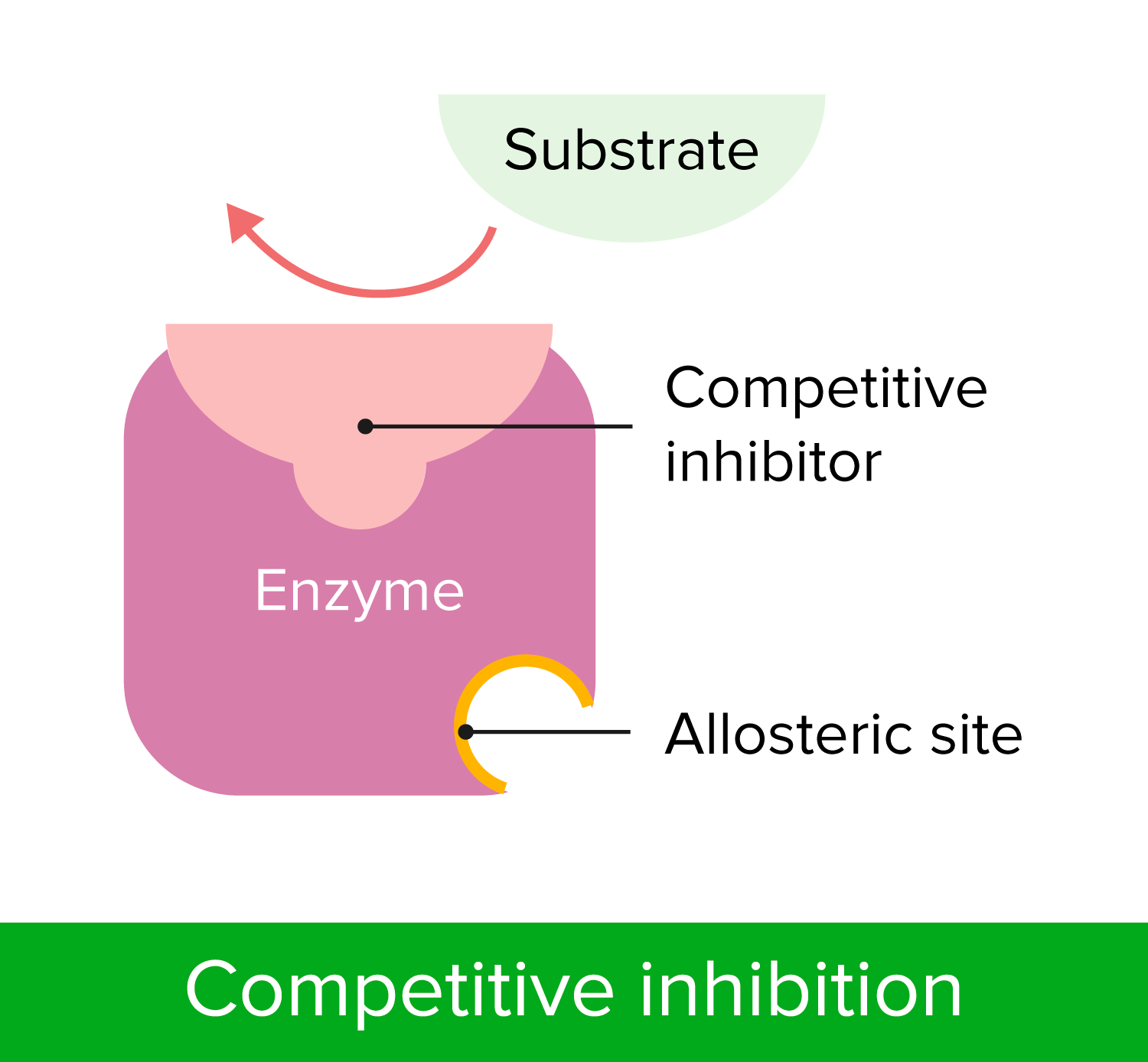
00:01 Zu verstehen, wie Enzyme gehemmt werden hat wichtige Auswirkungen sowohl auf unser Verständnis der Mechanismen der enzymatischen Wirkung als auch auf medizinische Überlegungen. 00:09 In diesem Vortrag werde ich hauptsächlich über zwei Dinge sprechen. 00:12 Reversible Enzyminhibitoren und auch irreversible Enzyminhibitoren. 00:18 Zellen sind natürlich auf Enzyme angewiesen, um Reaktionen zu katalysieren. 00:22 Und diese Abhängigkeit von Enzymen erlaubt uns, Zellen zu kontrollieren, wenn wir die Enzyme kontrollieren können. Dies machen wir uns zunutze insbesondere, wenn wir beispielsweise ein Bakterium haben, das wir daran hindern wollen, etwas zu infizieren, oder eine Krebszelle, die wir an der Ausbreitung hindern wollen. 00:38 Die Hemmung von Enzymen ist also ein wichtiger Aspekt für gesundheitliche Zwecke. 00:43 Ich möchte etwas Zeit darauf verwenden, über drei verschiedene Arten der Hemmung von Enzymen zu sprechen. 00:48 Und die erste dieser Möglichkeiten, über die ich sprechen werde, ist die sogenannte kompetitive Hemmung. 00:52 Sie können dies schematisch auf dem Bildschirm sehen. Das Enzym mit seinem normalen Substrat ist links dargestellt. 00:57 Das Enzym bindet an das Substrat und wandelt das Substrat in ein Produkt um. 01:02 Auf der rechten Seite sehen wir dasselbe Enzym, das gleichzeitig das Ziel eines Hemmstoffs für dieses Enzym ist. 01:07 Und in diesem Fall sieht der Hemmstoff des Enzyms wie das ursprüngliche Substrat aus. 01:12 Er passt in das aktive Zentrum des Enzyms. 01:15 Auf die gleiche Weise wie das normale Substrat. 01:18 Aber der Inhibitor hat etwas an sich, das das Enzym nicht manipulieren kann. Es kann nichts mit ihm anfangen. 01:24 Dadurch dreht das Enzym gewissermaßen erfolglos seine Räder, während es an den Inhibitor gebunden ist. 01:30 Dieser Inhibitor wird als kompetitiver Inhibitor bezeichnet und der kompetitive Inhibitor besitzt die Eigenschaften, die ich eben gezeigt habe: Dass er wie das Substrat aussieht und an das aktive Zentrum bindet. 01:40 Nun können Sie auf dem Bildschirm ein paar verschiedene Moleküle sehen. 01:45 Das untere Molekül ist ein Molekül, das von einem Enzym namens Dihydrofolatreduktase umgesetzt wird. 01:51 Das Enzym Dihydrofolatreduktase nutzt dieses Molekül und wandelt es in ein Produkt um, und dieses Produkt wird zur Herstellung von Nukleotiden verwendet - sehr wichtig für Nukleotide. 02:02 Das Molekül darüber heißt Methotrexat. Und Methotrexat ist dem Dihydrofolat sehr ähnlich. 02:10 Allerdings besitzt es einen wichtigen Unterschied. Und dieser Unterschied verhindert, dass das Enzym Dihydrofolatreduktase wirken kann. 02:17 Nun, Methotrexat ist ein Inhibitor dieses Enzyms, und durch die Hemmung eines Enzyms, das Nukleotide herstellt und spezifisch für eine Zelle ist, könnte man sich vorstellen, dass man diese Zelle an der Teilung hindern könnte. 02:27 Und das ist genau das, wofür dieser Inhibitor eingesetzt wird. 02:31 Untersuchen wir nun die Auswirkungen dieses kompetitiven Inhibitors auf ein Enzym. 02:35 Wenn wir ein Enzym nehmen und das V/S Diagramm einer ungehemmten Reaktion mit einer gehemmten Reaktion vergleichen, erhalten wir etwas wie das, was wir hier auf dem Bildschirm sehen. 02:45 Jetzt muss ich erklären, wie das gemacht wurde. 02:47 Ich habe beschrieben, wie wir - sagen wir 20 Röhren benutzt haben, um die Daten für die die erste Kurve zu generieren. Das Enzym plus unterschiedliche Mengen Substrat - jedes Röhrchen hat eine andere Menge Substrat - und ein Puffer werden verwendet und wir messen die Reaktionsgeschwindigkeit durch Messung der Konzentrationen der erzeugten Produkte im Laufe der Zeit. 03:06 Wenn wir die Hemmstoffreaktion untersuchen wollen, wollen wir uns daran erinnern, dass wir eine Variable suchen. 03:14 Und die eine Variable, die wir schon haben ist die Substratkonzentration. 03:17 Das bedeutet, dass wir nicht die Menge des Inhibitors variieren dürfen. 03:21 Wenn wir also den zweiten Reaktionsansatz durchführen, haben wir die gleiche Menge Enzym, wir haben den gleichen Puffer und wir haben die gleiche Menge Inhibitor in jedem Röhrchen. 03:29 Aber wir haben unterschiedliche Mengen Substrat. 03:32 Was passiert, wenn wir das tun? Nun, wenn wir das tun sehen wir, dass die Reaktion mit einer geringeren Geschwindigkeit anfängt. 03:38 Das ist nicht allzu überraschend, denn es gibt dort einen Inhibitor, der das Enzym hemmt. Die Geschwindigkeit ist niedriger. 03:43 Aber wenn wir die Substratmenge erhöhen, sehen wir, dass die Geschwindigkeit steigt und steigt und steigt, und am Ende erreicht sie tatsächlich den Bereich der Geschwindigkeit der ungehemmten Reaktion, okay? Wir sehen, dass die Differenz zwischen den beiden Kurven abnimmt. 04:01 Nun, ich komme jetzt zur Sache und werde Ihnen sagen, dass wenn wir sehr große Mengen Substrat verwenden, werden wir entdecken, dass die beiden Enzyme die gleiche Vmax haben. 04:12 Warum ist das der Fall? Warum hat eine kompetitiv gehemmte Reaktion die gleiche Vmax wie eine komplett ungehemmte Reaktion? Die Antwort liegt darin begründet, wie das Experiment aufgebaut war. 04:23 Ich sagte, dass wir eine feste Menge Inhibitor hatten. 04:26 Bei gigantischen Konzentrationen von Substrat: was passiert? Nun, das Substrat wird viel wahrscheinlicher vom Enzym gefunden als dass der Inhibitor vom Enzym gefunden wird. 04:36 Bei niedrigen Konzentrationen konkurrieren sie ziemlich stark. 04:40 Aber bei hohen Konzentrationen, wo ich vielleicht eine Million mal so viel Substrat wie Inhibitor habe, ist der Unterschied zwischen dem Ungehemmten und dem Gehemmten für mich schwer zu erkennen. 04:50 Neben der Tatsache, dass sich Vmax bei einer kompetitiv gehemmten Reaktion nicht ändert, ändert sich dafür etwas anderes an der Reaktion, und zwar der Km-Wert. 04:59 Da die beiden Reaktionen, d.h. die ungehemmte und die gehemmte Reaktion die gleiche Vmax haben, haben sie auch die gleiche Vmax/2. 05:07 Wenn wir also auf jeder Kurve den Km-Wert eintragen, den wir aus Vmax/2 erhalten entdecken wir, dass der Km-Wert für die nicht gehemmte Reaktion so groß wäre wie erwartet. 05:17 Aber der Km für die kompetitive gehemmte Reaktion erhöht sich. 05:22 Nun, dieser Anstieg deutet auf eine scheinbare Veränderung der Affinität des Enzyms für das Substrat hin. 05:27 Ich sage "scheinbar", denn es ändert sich eigentlich nicht die Affinität des Enzyms für das Substrat. Das ist ein tieferes Thema als ich hier bespreche. Aber der scheinbare Km steigt, was den Anschein erweckt, dass das Enzym seine Affinität für sein Substrat verliert. 05:43 Dies kann auf eine andere Weise grafisch dargestellt werden: mit Hilfe eines Lineweaver-Burk-Diagramms. 05:47 Erinnern Sie sich: Beim Lineweaver-Burk-Diagramm nehmen wir dieselben Werte, die wir für das V/S-Diagramm hatten. 05:51 Wir bilden den Kehrwert aller Daten und stellen diese dann in einem reziproken Diagramm dar, wie Sie hier sehen: 1/Vo auf der Y-Achse und 1/ Konzentration von S auf der x-Achse. 05:59 Wenn wir das tun, sehen wir, dass - nicht überraschend - die V/S-Daten eine Gerade ergeben, wie hier in grün dargestellt, wobei der Schnittpunkt mit der y-Achse 1/Vmax entspricht und der Schnittpunkt mit der x-Achse -1/Km. 06:13 Wenn wir den kompetitiven Inhibitor darstellen, sehen wir genau das, was wir in unserem letzten Diagramm gelernt haben, nämlich dass Vmax gleich ist und dass die beiden Geraden sich auf der y-Achse schneiden. 06:22 Und da sich der Km-Wert für die kompetitive Hemmung erhöht hat, sehen wir dann, dass -1/Km sich 0 nähert. 06:31 Das Lineweaver-Burk-Diagramm zeigt uns sehr sehr anschaulich, was bei dieser Hemmung passiert.
About the Lecture
The lecture Competitive Reversible Enzyme Inhibition – Enzyme Inhibitors by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
What happens to the Km value of an enzyme in the presence of a competitive inhibitor?
- It increases.
- It decreases.
- It remains unaffected.
- It decreases by half.
- It decreases by 10%
Which of the following statement is NOT true regarding competitive reversible enzyme inhibition?
- In competitive reversible enzyme inhibition, the enzyme participates in a new chemical reaction involving the conversion of the inhibitor molecule to the product instead of using the original substrate.
- In a competitive reversible enzyme inhibition, the Vmax and 1/Vmax values do not change in the presence of an inhibitor during an enzymatic reaction.
- At very low substrate concentration, the competitive inhibitor has a more pronounced effect on the rate of enzymatic reaction than at higher substrate concentrations.
- Competitive inhibition of an enzyme is used in the treatment of some cancers.
- The competitive inhibitor resembles the original substrate and hence easily fits into the active site of the enzyme.
Which of the following acts as a competitive inhibitor for the enzyme dihydrofolate reductase?
- Methotrexate
- Dihydrofolate
- Nucleotides
- Penicillin
- Isoleucine
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |