Playlist
Show Playlist
Hide Playlist
Common Sugars: Nomenclature & Structure – Simple Carbohydrates
-
06 Basic SimpleCarbohydrates V2.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
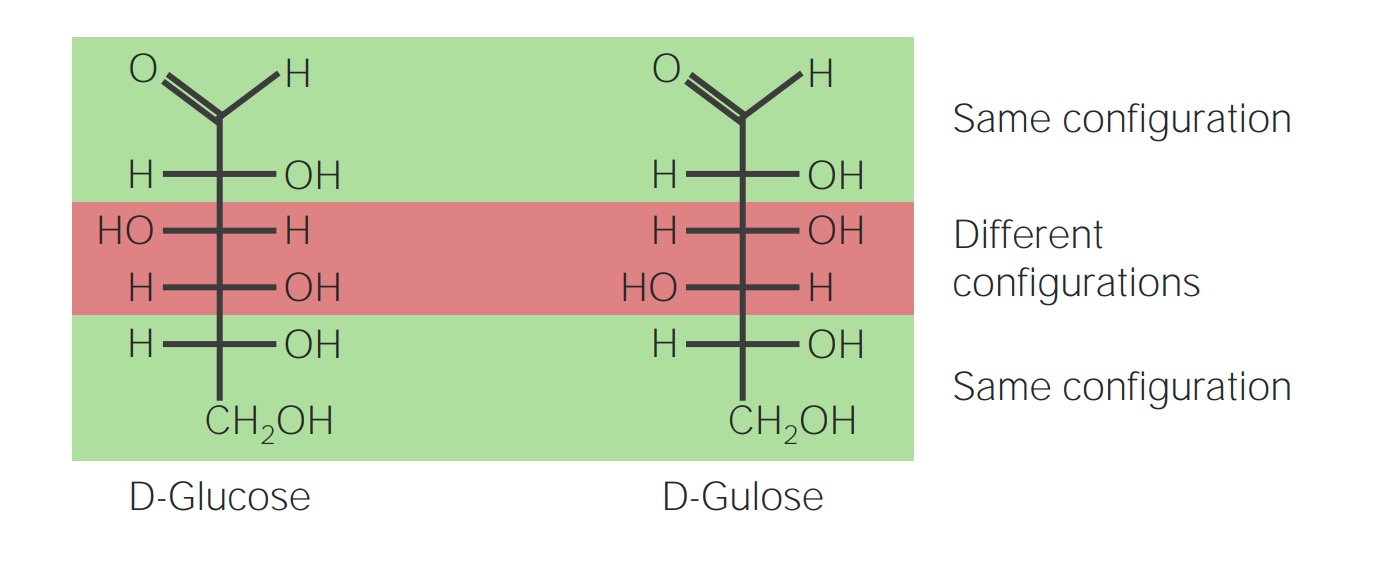
00:01 Jetzt gibt es eine Menge Nomenklaturen, die mit den Zuckern verbunden sind und ich möchte diese mit Ihnen durchgehen, weil diese allgemein zur Beschreibung verwendet werden, um die Zucker und ihre Eigenschaften zu beschreiben. 00:11 Die ersten Begriffe, die ich einführen möchte, sind die von Aldose und Ketose. Um sie einzuführen, muss ich Ihnen zwei Moleküle präsentieren, nämlich die Moleküle von Glukose und Fruktose, zwei gewöhnlichen Zuckern, die wir in der Natur finden. Wenn wir die Strukturen dieser beiden Moleküle analysieren, sehen wir, dass für die unteren vier Kohlenstoffe jedes dieser Moleküle identische Strukturen hat. 00:31 Sie haben das obere mit dem Hydroxid auf der linken Seite, die nächsten beiden Hydroxide auf der rechten Seite und sie enden in einem CH2OH. Allerdings sind die ersten beiden Kohlenstoffe dieser Moleküle unterschiedlich. 00:43 Wir sehen zum Beispiel, dass Glukose ganz oben in einer Aldehydgruppe endet, während Fruktose kein Aldehyd ist, sondern eine Ketongruppe an Kohlenstoff Nummer zwei hat. 00:54 Das bedeutet, dass Glukose das ist, was wir als eine Aldose bezeichnen, also ein Aldehyd-Zucker, und Fruktose ist eine Ketose, also ein Ketosezucker. Ich sollte auch erwähnen, dass immer, wenn wir den Namen Zucker verwenden, wir die Buchstaben OSE ans Ende des Namens setzen, um anzuzeigen, dass es sich um eine Form eines Kohlenhydrats handelt. 01:13 Die verschiedenen Zucker, die es gibt, existieren in einer Vielzahl von Formen, Gestalten und Größen. In diesem Fall vergleichen wir die unterschiedlichen Größen der einzelnen Zuckerformen. In diesem Fall habe ich vier verschiedene Aldosen. Das erste dieser Glyceraldehyde hat drei Kohlenstoffe und wir beschreiben es als eine Triose. Das zweite hat vier Kohlenstoffe und wir bezeichnen es als Tetrose. Der dritte, Ribose, ist ein häufiger Zucker in Nukleotiden, hat fünf Kohlenstoffe und ist eine Pentose. Und der letzte, Glukose, hat sechs Kohlenstoffe und ist eine Hexose. Wir können die Namen kombinieren, wir können zum Beispiel Glukose als Aldohexose beschreiben. 01:56 Neben der Größe gibt es auch Unterschiede zwischen den einzelnen Zuckern in Bezug zur Stereochemie. Nun zurück zur organischen Chemie. Wir erinnern uns, dass jeder Kohlenstoff, der an vier verschiedene Atome gebunden ist, diese vier Atome in dreidimensionaler Anordnung auf zwei verschiedene Arten haben kann. Was ich Ihnen also auf dem Bildschirm zeige, sind zwei verschiedene Formen des Einfachzuckers Glyceraldehyd. Sie können sehen, dass der zentrale Kohlenstoff in diesem Fall asymmetrisch ist; er hat vier verschiedene Moleküle angehängt. Die L-Konfiguration wird verwendet, um zu kennzeichnen, dass sich das Hydroxid auf dem linken Teil des Moleküls befindet, wie man hier sieht, und die D-Konfiguration wird verwendet, um das Molekül zu beschreiben, wenn es das Hydroxid an dem rechten Teil hat. Jetzt werden wir sehen, dass Zucker höherer Ordnung, d.h. größere Zucker, mehrere asymmetrische Zentren haben. Die Bezeichnungen D und L geben nur die Konfiguration für das asymmetrische Zentrum am unteren Ende des Zuckers an, wenn er in der linearen Form geschrieben wird, wie wir noch sehen werden. D und L Glyceraldehyd haben eine andere Eigenschaft, die mit ihnen verbunden ist und sie sind spiegelbildlich zueinander, was, wie wir sehen werden, einen besonderen Namen bekommt. 03:11 Wie ich bereits sagte, haben einige Zucker mehrere asymmetrische Zentren. Glukose hat zum Beispiel vier verschiedene asymmetrische Zentren, wie hier gezeigt. Jedes dieser Kohlenstoffe kann in verschiedenen Konfigurationen vorliegen und wenn wir eine andere Konfiguration haben, haben wir keinen Traubenzucker mehr, also ist die Benennung nicht bestimmt von der Stereochemie, sondern von den einzelnen gebräuchlichen Namen der Zucker. Mit vier asymmetrischen Zentren, kann eine Aldose 16 verschiedene Namen haben, die mit ihr verbunden sind. 03:45 Dieser Zucker ist D-Glucose, weil er neben dem letzten Kohlenstoff, ganz unten, ein Hydroxid auf der rechten Seite hat, wie für Glyceraldehyd angegeben. Es ist also der nächste oder letzte Kohlenstoff, der die D-Konfiguration gegenüber der L-Konfiguration bezeichnet. Wenn wir das Spiegelbild der D-Glucose nehmen würden, hätten wir die so genannte L-Glukose. Die Bezeichnung L kommt nicht von ungefähr, nicht etwa einfach durch Umdrehen des Hydroxids auf dem unteren Kohlenstoff, stattdessen kehrt das Spiegelbild von D-Glukose jede einzelne um. Wenn wir also D-Glukose betrachten, sehen wir, dass sie am Kohlenstoff Nummer 2, die Hydroxide auf der rechten Seite, dann auf der linken Seite und dann die rechte Seite auf die linke richtet. Aber wenn wir uns L-Glukose ansehen, ist jedes dieser Elemente umgedreht, beginnend bei der Kohlenstoffnummer 2, links, rechts und dann links, links. Der Begriff, den wir zur Beschreibung von Zuckern verwenden, die spiegelbildlich zueinander sind, ist der Begriff Enantiomer. Es ist wichtig zu beachten, dass jeder D-Zucker sein Spiegelbild mit den gleichnamigen L-Zucker mit dem gleichen Namen hat. Also D-Glucose-Enantiomer ist L-Glucose, D-Glyceraldehyd Enantiomer war L-Glyceraldehyd. Ähnliches gilt für Fructose. 04:58 Das Enantiomer der D-Fructose ist die L-Fructose. 05:01 Ein weiterer wichtiger Begriff, den man verstehen muss, ist der Begriff Diastereomere. Diastereomere, wie wir in der Struktur hier sehen, beziehen sich auf Zucker, die unterschiedliche Konfigurationen haben und die die gleiche Grundchemie besitzen, aber keine Spiegelbilder des anderen sind. Was Sie hier sehen, ist D-Glucose und ein verwandter Zucker namens D-Gulose. D-Glukose und D-Gulose haben eine ähnliche Struktur. 05:24 Zum Beispiel sind beide Aldosen, wie Sie an ihren Aldehydgruppen oben sehen können. Sie können auch sehen, dass ihre Hydroxide an Kohlenstoff Nummer zwei sind, beide sind auf der rechten Seite. Unten, auf der rechten Seite steht Hydroxid, gefolgt von einem CH2OH. Wenn wir jedoch die Strukturen der Kohlenstoffe an den Positionen drei und vier vergleichen, sehen wir, dass sie nicht identisch sind. Und D-Glucose und D-Gulose sind demnach Diastereomere. Diastereomere, der Begriff, bezieht sich auf Zucker, die unterschiedliche Konfigurationen und die gleiche Chemie haben, also Aldosen in diesem Fall, aber nicht spiegelbildlich zueinander sind. Und Sie können den Unterschied genau dort in ihren verschiedenen Konfigurationen hervorgehoben sehen. 06:08 Ein Diastereomer, ein spezieller Typus eines Diastereomers, ist ein Molekül, dass ein Epimer genannt wird. Ein Epimer ist also ein Diastereomer, das nur einen einzigen Kohlenstoff hat, dessen Konfiguration unterschiedlich ist. Wenn wir also zum Beispiel D-Glukose und D-Galaktose vergleichen, sehen wir, dass sie identische Strukturen in Bezug auf ihrer Hydroxidgruppen haben, mit Ausnahme des Kohlenstoffs. 06:32 Das ist in orange dargestellt.
About the Lecture
The lecture Common Sugars: Nomenclature & Structure – Simple Carbohydrates by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following statements is NOT true?
- Ribose is an aldo-hexose
- D-glucose is the enantiomer of L-glucose.
- Glucose is an aldo-hexose.
- Fructose is a keto-hexose.
- Glyceraldehyde is an aldo-triose.
The term enantiomers refers to which of the following?
- Two sugars that are mirror images of each other
- Two sugars that differ in configuration of one carbon
- Two sugars that differ in configuration of their anomeric carbons
- An aldose-ketose pair, such as fructose and glucose
- Two monosaccharides that form a disaccharide
What are α-D-glucose and α-D-galactose?
- Epimers
- Aldose-ketose pairs
- Mirror images
- Enantiomers
- Polymers
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Concise and clear; exactly what I needed for this topic, thank you.