Playlist
Show Playlist
Hide Playlist
Citric Acid Cycle
-
Slides CitricAcidCycle.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
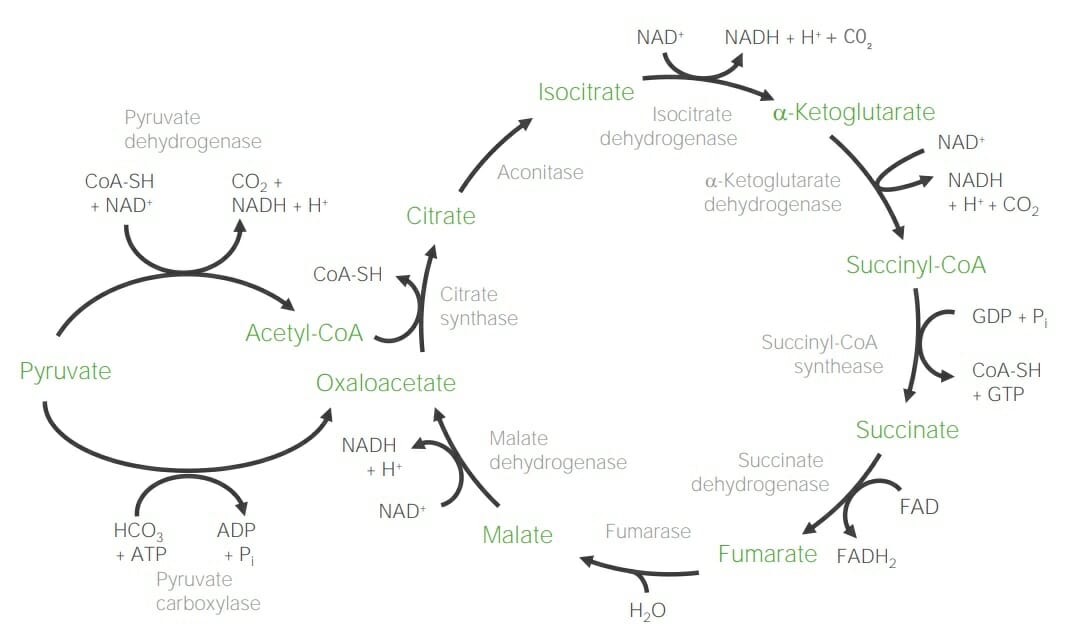
00:01 Next, I wanna talk about the citric acid cycle. 00:04 Now the citric acid cycle, as I’ve noted, is the most important oxidative cycle in the body. 00:10 The cycle is a true cycle. 00:13 It goes over and over, round and round needing only input from an acetyl-CoA at each round of the cycle. 00:21 Now acetyl-CoA, as noted, can be produced from pyruvate by action of the enzyme pyruvate dehydrogenase. 00:29 Pyruvate is a very versatile molecule and if necessary, pyruvate can also be made into oxaloacetate using catalysis of the enzyme pyruvate carboxylase as it’s been noted in another lecture on gluconeogenesis. 00:44 Now, what I wanna do here is step through each of the individual reactions of the citric acid cycle to tell you something about each one of them. 00:54 There is not a beginning or the end to the citric acid cycle because it is, of course, a cycle. 01:00 But the most common place that people refer to as the entry point or the beginning of the citric acid cycle is the entry of acetyl-CoA as shown here. 01:09 This reaction is catalyzed by the enzyme known as citrate synthase and it is a very energetic reaction producing a lot of energy. 01:19 This happens because the bond between the coenzyme A and the acetyl group and acetyl-CoA releases energy and that’s necessary to release the acetyl groups that it can combine with the oxaloacetate to form citrate. 01:35 This results in a reaction having very negative delta G zero prime value and this turns out to be important when we get all the way around the cycle because after we’ve gotten around the cycle we’ll see that the enzyme that makes oxaloacetate, in fact, is not energetically favorable and this reaction helps to pull it forward. 01:56 The next step of the cycle is a reaction catalyzed by the enzyme known as aconitase. 02:04 Now this reaction is a very simple one. 02:06 All that’s happening in the reaction is a rearrangement of the molecule of citric acid. 02:12 In this case, the hydroxyl group shown on carbon number three on the left is moved to carbon number four as shown on the right. 02:21 In the next step of the cycle, the enzyme isocitrate dehydrogenase catalyzes the first of two decarboxylations that occur in the citric acid cycle. 02:31 In this reaction, the carboxyl group shown as carbon number six on the citrate molecule on the left is lost converted into carbon dioxide; we see its absence on the right. 02:43 This reaction, this decarboxylation, is an oxidation and the electrons of the oxidation are transferred to NAD to form NADH. 02:54 The product of the last reaction was a molecule known as alpha-ketoglutarate; it’s shown on the left in this slide. 03:01 And in this slide we see that alpha-ketoglutarate is itself decarboxylated and oxidized to form a molecule known as succinyl-CoA. 03:11 The enzyme catalyzing this reaction is known as alpha-ketoglutarate dehydrogenase. 03:16 And again, as we saw before, carbon dioxide is a product of the reaction. 03:22 NADH is a product because the electrons are transferred to NAD. 03:26 And in addition, a CoA is attached to the succinyl group that’s made to make succinyl-CoA. 03:33 Now this turns out to be important because this CoA bond to the succinate is in fact a very high energy bond as we’ll see in the next reaction. 03:43 In the next reaction, succinyl-CoA is converted into succinate. 03:48 Now this is a reaction that releases energy as I will describe in just a minute. 03:52 The enzyme catalyzing this reaction is known as succinyl-CoA synthetase and it would sound from the name of the enzyme like it’s making succinyl-CoA. 04:02 Well in fact, the enzyme was named by people who were studying the reaction that was going in the backwards direction that is the synthesis of succinyl-CoA. 04:11 We’re interested, however, in the reverse reaction and since enzymes catalyze reverse reactions it’s important to understand why the naming is as it is. 04:20 This reaction is the only reaction of the citric acid cycle that results in the formation of a triphosphate directly that is what’s known as a substrate level phosphorylation and you can see it in the conversion of GDP to GTP. 04:36 Some organisms I know use ADP and convert that into ATP so that’s why I call it the formation simply of a triphosphate. 04:45 Now, the energy necessary to make this triphosphate is realized as a result of breaking of the bond between the coenzyme A and the succinate, resulting in the formation of free succinate and free CoA and of course the energy is used to make GTP. 05:03 Now, succinate dehydrogenase catalyzes the next reaction and this is the first of three reactions that are chemically identical to the first three reactions of beta-oxidation of fatty acids. 05:18 In this reaction, we see that electrons from the middle two carbons of succinate are removed forming a double bond. 05:26 This results in the formation of a molecule known as fumarate and in the process electrons are transferred to FAD to form FADH2. 05:36 If you’re keeping track, this is the third oxidation of the citric acid cycle. 05:41 Now, succinate dehydrogenase is an interesting enzyme. 05:45 It is embedded in the membrane of the inner mitochondria. 05:50 It is the only enzyme of the citric acid cycle that is not found dissolved in the matrix. 05:55 And in fact, succinate dehydrogenase is also known as complex two in the process of electron transport. 06:03 Now we can actually see that happening here. 06:06 At the bottom of the slide we see the oxidation of succinate to form fumarate, the formation of FADH2 with the transfer of electrons to FAD. 06:16 Within the succinate dehydrogenase complex, there are four different regions labeled as SDHA for succinate dehydrogenase-A, SDHB, SDHC and SDHD. 06:31 Now we like to follow this in detail but the electrons travel through the various subunits with the assistance of a heme. 06:38 And ultimately, these electrons are transferred to coenzyme Q to form reduced coenzyme Q shown as QH2. 06:46 This then moves through the membrane of the mitochondrion in the electron transport system. 06:51 Coming back to the citric acid cycle we see the next step in the process. 06:55 In this reaction, the enzyme fumarase catalyzes the addition of water to the double bond shown in the fumarate on the left to form the molecule of malate shown on the right. 07:08 Now, this reaction is very similar to the second step in the oxidative process of fatty acids. 07:14 In that reaction, a very similar thing happens, water is added and a molecule with the hydroxyls formed as shown on the right. 07:22 Now, the malate that’s formed in the last reaction is the substrate of the enzyme for this reaction. 07:29 The enzyme malate dehydrogenase catalyzes the conversion of malate into oxaloacetate and this is the fourth and last oxidation of the citric acid cycle, and of course, electrons are transferred to NAD to form NADH as shown in the figure. 07:47 Now one of the things about this reaction is that it’s a very unusual oxidative reaction and that it has a fairly positive delta G zero prime, meaning, it’s energetically not very favorable in the forward direction. 08:00 And it’s this reaction that I was referring to in the citrate synthase reaction where this reaction depends on the energy produced in the following reaction by the citrate synthase to help pull this reaction forward and help keep the citric acid cycle moving. 08:17 So an overview of the citric acid cycle is shown here and you can see the overall process with each of the individual oxidations that I’ve described and the feeding of the cycle by the acetyl-CoA from the pyruvate. 08:31 In summary, the citric acid cycle takes two carbon seen in the form of one acetyl-CoA. 08:37 It releases two carbon dioxide molecules; so two carbons in, two carbons out. 08:43 It has four oxidations and produces three molecules of NADH, one molecule of FADH2 and one GTP per turn of the cycle. 08:54 These are very important energy sources for cells. 08:58 Now, as I noted earlier, the intermediates of the citric acid cycle are very important for cells to use for other things and I’ve summarized a bit of that in this slide here. 09:07 The molecule citrate we can see is used in the glyoxylate cycle and it has also an allosteric effector affecting other enzymes. 09:14 It also is involved in shuttling molecules of acetyl-CoA out of the mitochondria needed to make fatty acids. 09:22 Isocitrate is an intermediate that’s important in the glyoxylate cycle. 09:26 Alpha-ketoglutarate is very important for amino acid and nitrogen metabolism. 09:31 Succinyl-CoA is used to make heme that’s important for the formation of hemoglobin in our blood cells. 09:39 It’s also very important in amino acid metabolism. 09:42 Succinate is necessary for glycogen metabolism, odd chain fatty acid metabolism that occurs in some unusual reactions. 09:51 Fumarate is necessary for glyoxylate metabolism and also for nucleotide metabolism, and malate is necessary for the glyoxylate metabolism and also the shuttle of electrons across the mitochondrial membrane. 10:05 And last, oxaloacetate is found almost everywhere. 10:08 Some of the places where it’s found are in glyoxylate metabolism, in gluconeogenesis and also in amino acid metabolism.
About the Lecture
The lecture Citric Acid Cycle by Kevin Ahern, PhD is from the course Carbohydrate Metabolism.
Included Quiz Questions
Which of the following is not correctly matched?
- Malate — generates FADH2 when it is converted to oxaloacetate
- Citrate — allosteric effector for some enzymes
- α-Ketoglutarate — used in amino acid and nitrogen metabolism
- Succinyl-CoA — used to make heme
- Fumarate — important in nucleotide metabolism
Which of the following is correct regarding the energy output per cycle of the citric acid cycle?
- 3 NADH, 1 FADH2, and 1 GTP/ATP
- 1 NADH2, 3 FADH2, and 1 ATP
- 1 NADH2, 1 FADH2, and 1 ATP
- 1 NADH2, 1 FADH2, and 3 GTP
- 1 NADH2, 3 FADH2, and 3 ATP
Which of the following enzymes of the citric acid cycle is embedded in the inner mitochondrial membrane?
- Succinate dehydrogenase
- Aconitase
- Isocitrate dehydrogenase
- Malate dehydrogenase
- α-ketoglutarate dehydrogenase
How many oxidation reactions take place in the citric acid cycle?
- Four
- One
- Two
- Three
- Five
Which of the following enzymes participates in both the TCA cycle and the ETC?
- Succinate dehydrogenase
- Fumarase
- Isocitrate dehydrogenase
- Malate dehydrogenase
- α-Ketoglutarate dehydrogenase
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
3 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
I can easily follow the sequence of information. The important details are also emphasized, making it easier for recall.
Very clear and easy to understand. I enjoyed the visuals.
1 customer review without text
1 user review without text